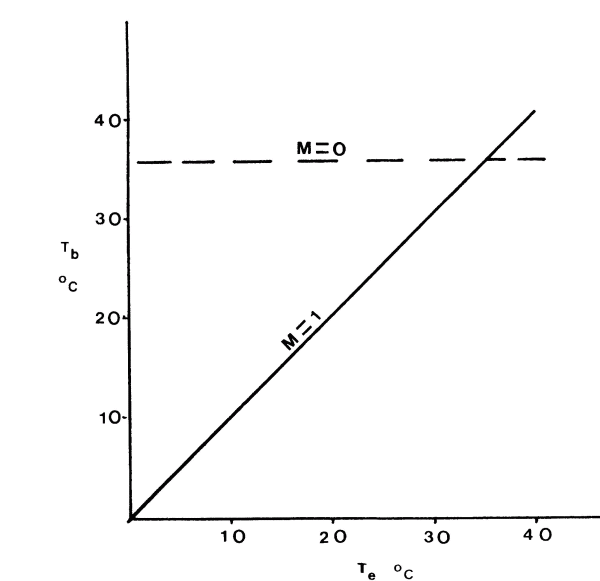ROGER MEEK
Huddersfield, Yorkshire, UK.
Introduction
Since the early pioneering studies of Strelnikov (1934) Sergeyev (1939) and Cowles and Bogart (1944) thermoregulation in reptiles has been recognised as a key element in their biology and extensively studied. Numerous reviews of these works have been published, the most recent and comprehensive (Avery, 1982) discussed the thermal relations of over 500 species. Much of the work on reptile thermoregulation has concerned lizards but other groups including chelonians have been investigated and several reviews published (eg Hutchison 1979; Sturbaum 1982; Meek & Avery 1988).
This paper looks at ways thermoregulating reptiles interact with their environments. It also examines how environmental constraints set fundamental limits on, for example, behavioural repertoires, thermal set points and distribution patterns. The main purpose is not to attempt a comprehensive review of the subject since space does not permit what would be a major undertaking, but to provide an insight into the subject using selective examples of research (citing chelonians when appropriate) to highlight adaptations and problem areas.
It is hoped that an insight into the thermal ecology of free-living reptiles will enable herpetologists involved in captive breeding projects to enhance captive environments. For example Avery (1985) and Avery & Bond ( 1 987) demonstrated that in thermoregulatory arenas, lizard foraging activity increased with increasing spatial heterogeneity, and Hailey (1982) observed that common lizards (Lacerta vivipara) switched from basking on grass to wood during cloudy weather to enhance heating rates. Therefore although other factors such as space, humidity, and nutrition are involved in captive breeding, thermal distribution patterns and substrate materials, particularly in outdoor enclosures, are important factors to be taken into account in reptile husbandry.
Thermoregulation; the key to reptile biology
Thermoregulatory behaviour is not an end process in itself but a critical mechanism that enables reptiles to enhance physiological performance i.e. running speeds, growth, reproduction and digestion, by exploiting thermal distribution patterns in the environment to attain physiological optimum body temperatures. The concept of a physiological optimum temperature is related to the body temperature which a reptile will often select, given a range of environmental thermal zones from hot to cold. These are known as the preferred or eccritic body temperatures and are the temperatures that the physiological processes are said to work best. The progression of efficiency between the increments in temperatures toward the physiological optimum temperature can be most clearly expressed in terms of a temperature coefficient, the Q10 value, derived from the equation,
Q10 = (K1/K2)10/( t1/t2)
where K1 and K2 are the velocity constants proportional to the rates of reaction observed at temperatures t1 and t2. Very simply this equation gives the predicted increase in physiological processes for every increase in body temperature of 10°C. Most biological reactions have Q10"s of 2-3; for example a Q10 of 2 for muscular energy between 20 - 30°C indicates an increase of twice the amount of energy available in the muscles over that particular increase in temperature range.
There is, as well as thermal set points where physiological processes work optimally, an optimum range of body temperatures that reptiles may employ. Optimum ranges fluctuate between lower and upper thermal thresholds and not around a central point in temperature. In addition to ecological factors, optimum temperature ranges can also be influenced by the physiological state of the animal at any given time, for example digestion (Gatten, 1974) or reproductive condition (Obbard & Brooks, 1979): this has led to the hypothesis that there are multiple physiological optimum temperatures, although Huey (1982) has drawn attention to the inherent difficulties in this theory. Seasonal differences in preferred body temperatures and ranges have been recorded (Turner et al., 1976).
Reptiles are critically constrained by a series of body temperature thresholds, the ecological critical maximum or minimum body temperatures. These are the temperatures that because they bring about an absence of effective muscular coordination, restrict the reptile's ability to escape from conditions which will ultimately lead to its death. Ecological critical (maximum) temperatures may occur at temperatures close to operative field body temperatures. Hutchison (1979) gives a list (albeit a now somewhat outdated list) of chelonian body temperatures. In chelonians for example ecological maximum body temperatures occur around 41-43°C (Hutchison, Vinegar & Kosh, 1966). Death occurs at the physiological critical (lethal) maximum or minimum body temperatures. Critical thermal zones in body temperature ultimately set absolute limits on where or when reptiles can survive.
Beer cans. thermoconformers and thermoregulators
One of the early definitions of evidence of thermoregulatory behaviour in reptiles was if there was an elevation of body temperature above environmental temperatures. However in a famous " beer can " experiment Heath (I 964) showed that filled beer cans when placed out in the open also had temperatures higher than environmental temperatures and argued that such a definition of thermoregulation leads to the conclusion that either beer cans are able to thermoregulate or the method of observation can be misleading.
Eventually evidence of thermoregulation was defined as a regression of body temperature with air temperature by use of the regression equation,
y = mx + b
where the slope m relating the variables y (body temperature) and x (an environmental temperature) is expected to vary between 1 and 0. The value of b in the equation defines the y intercept, the starting point on the axis of the slope. Figure I shows this relationship. A slope with a value of 0 indicates a perfect thermoregulator since body temperature is completely independent of environmental temperature. The slope with a value of 1 indicates a thermoconformer since y is in agreement with x at all times; that is the animal is not attempting to thermoregulate but allowing its body temperature to closely track environmental temperatures.
Regression equations can however be fitted to data with almost any amount of scatter and a degree of intellectual judgment should be applied to their use. For example confidence intervals around factor m can be calculated defining the agreement between m and the distribution of its data base, and taken into account in any analysis.
More recently a new method has been proposed (Hertz 1992). Here the view is that thermoconformity will not necessarily indicate body temperatures equal to environmental temperatures and that the true measure should be between the body temperature the reptile has and that which it would have if it did not thermoregulate. To test this hypothesis, models are placed at random in the environment and their temperatures are compared with those of the animals. Huey (1982) discusses the various methods of estimating extent of thermoregulation and their limitations.
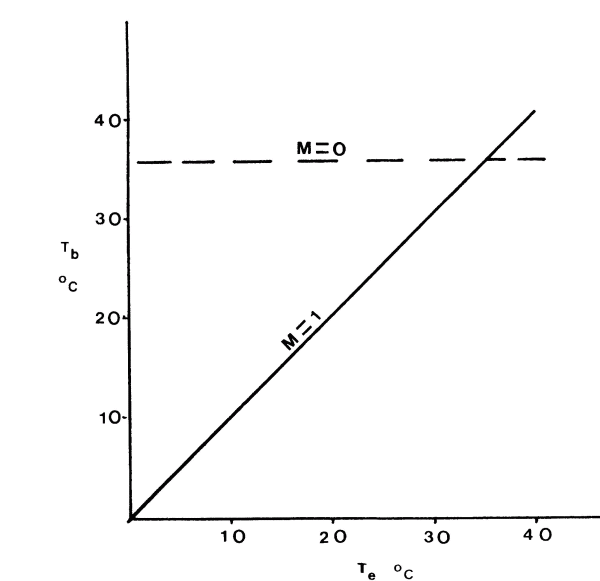
Figure 1. Definition of thermoregulation using the regression equation y = mx+b, where body temperature y is related to an environmental temperature x by the slope of the regression m and the y intercept b. A slope m with a value of 1 (solid line) defines a thermoconformer since the body temperatures are in agreement with some environmental temperature. A perfect thermoregulator's body temperature in independent of environmental temperature and defined by the broken line (m = 0).
Thermoregulatory strategy
The evolution of thermoregulatory behaviour in reptiles almost certainly arose because environments are not usually thermally stable but fluctuate both on a daily or seasonal basis or because of uneven heat distributions within environments. Seasonal or daily shifts in reptilian thermoregulatory effort to compensate would therefore have had selective advantages and although such adaptations have not been extensively studied, where they have been sought they have been found. The fact that physiological performance is optimal and profoundly influenced by temperature suggests that the precision with which a reptile thermoregulates should indicate the cost that the habitat and its climate imposes on precise thermoregulation; precise body temperatures should only be expected in environments that are thermally stable and the heat sources easily accessible (low cost habitat). In habitats where heat flow patterns are intermittent and/or difficult to access ( high cost habitats), reptiles should become imprecise thermoregulators and operate through the use of optimum ranges.
Therefore although a species may have a range of body temperatures for optimum physiological performance, they can only be considered optimal in an ecological context if the costs incurred in attaining them are low (Huey 1982). The optimum activity temperature(s) can be defined as the " ecological optimum", that which enables the reptile to maximise its energy intake with the greatest efficiency in any particular habitat (Huey & Slatkin 1976).
A theoretical model
Consider a hypothetical reptile, living in an ideal thermal environment where every day a broad range of heat sources are readily available for it to select from. In addition to a thermo-friendly environment the hypothetical reptile is free from predators or inter-specific competition and has a regular and easily accessible food source. Under such conditions physiological optimum body temperatures should be readily attained and hence the reptile could, through long daily spells at optimum temperatures, operate at maximum physiological capacity for prolonged periods.
Figure 2 is a theoretical cost-benefit model for a reptile faced with a shift towards a cooler climate. It shows the costs and benefits of retaining thermal set points or shifting to new lower thermal set points. The retention of thermal set points requires an increase in the amount of time spent basking with less time available for other ecological activities like feeding. This may not be required if adjustments are made to lower set points but physiological costs resulting in (for example) reduced running speeds are likely. This model is based on the "static" theory (Hertz et al., 1983); Crowley, 1985) where thermal benefits are believed fixed and must be retained if physiological performance is to be maintained. An alternative "labile" theory (Berkum, 1986; Huey & Bennet, 1987) argues that thermal optima are dynamic and will evolve with changing environments.
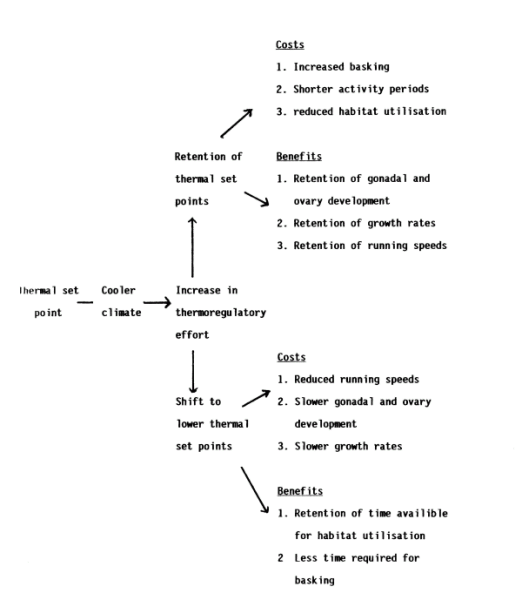
Figure 2. A graph showing a simplified theoretical cost-benefit analysis of the thermoregulatory options of a reptile experiencing a shift to a cooler climate. See text for details.
Reptiles in laboratory heat gradients.
Conditions approaching the optimum hypothetical situation actually exist. The experimental conditions designed to examine reptile thermal biology in laboratories where in thermal gradient chambers (usually a long enclosure with high temperatures at one end, graduating to cooler temperatures at the other end) reptiles may experience conditions approaching optimum thermal environments.
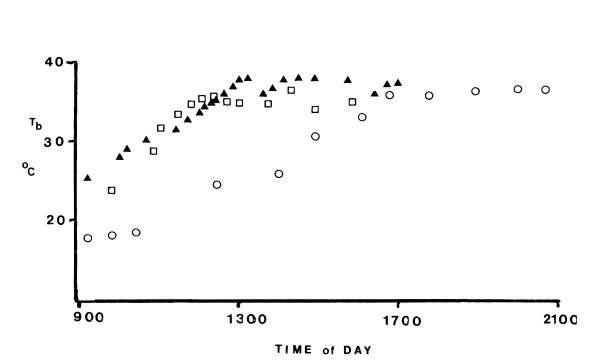
Figure 3 is a record of the body temperatures of two species of monitor lizard (Varanus) studied in a laboratory thermal gradient (Meek 1978). The graph shows that these large lizards (480g, 1.7kg) display the classical paradigm of heliothermy, that is, early morning basking produces an increase in body temperatures to around 38°C followed by fluctuations of around 2°C as a result of alternate periods of basking and movement behaviour. There are no predators, cloudy weather or the necessity to spend considerable time searching for food to interfere with thermoregulation. The only real variable (in this experiment) is between the two animals, the larger lizard because of its greater mass, and hence lower surface area to body mass geometry has slower heating and cooling rates. Altering the strength of the heating lamps may change the shape of the heating curves but has no significant influence on the temperatures the lizards maintain at the upper end of the curve-this has important implications for reptiles operating under natural conditions.
For a reptile to achieve optimum physiological body temperatures in natural environments an adequate supply of heat to the habitat is vital. The attainment of operative body temperatures is therefore primarily dependent on a number of important factors that include 1) the distribution of heat within the habitat 2) the animals' ability to gain access to the heat source (varying degrees of plant or cloud cover may restrict the heat rays' penetration through the habitat) 3) lifestyle, some species may be adapted to certain types of microhabitat which receive less amounts of heat ( e.g. fossorial species) 4) the qualitative nature of the thermal energy available, for example the way a reptile may be able to harness the sun's energy at high altitudes where there may be abundant sunshine, but very low ambient air temperatures, may require a quite different strategy to a habitat with a low number of sunshine hours but high ambient air temperatures.
Thermoregulation at high altitudes
Figure 3. Body temperature of monitor lizards (Varanus) in a laboratory heat gradient chamber. The largest lizard a 7Kg V. bengalensis (white circle) has a slower rate of heating that a 480g V. salvator (black triangle) and 1 Kg V. bengalensis (white square). Data has been redrawn from Meek(1978).
The most severe environments in which reptiles live are in high latitudes and altitudes. Thermal studies have taken place in both types of habitat, but perhaps the most remarkable were in the Peruvian Andes on the lguanid lizard Liolaemus multiformis above 4500m (Pearson, 1954). In this region even summer climates are characterised by near freezing air temperatures often with snow on the ground. However there are usually thick clumps of vegetation which enabled the lizards to insulate themselves from cold soil temperatures and clear sunny skies - albeit with frequent snow storms. By basking on the thick vegetation mats the lizards, even when emerging from their burrows when air temperatures were as low as - 5°C, in two hours or so, elevated their body temperatures to around 34°C. Depending on the frequency of the snow storms, Liolaemus could maintain high body temperatures for a good part of the afternoon (Pearson, 1954).
At an altitude of 4100m in the Caucasus in similar harsh climatic conditions the Lacertid lizard Lacerta agilis also displayed similar thermoregulatory adaptations attaining body temperatures of almost 30°C above ambient air temperatures (Strelnikov 1944). On the Mediterranean island of Corsica Bauwens et al., (1990) recorded body temperatures of 35°C in the Lacertid Lacerta bedriagae at altitudes of 1750-1800m. Precise thermoregulation in a high cost habitat was apparently achieved by subtle use of the environment such as using the abundant basking sites that were in full sun and quickly retreating to shelter when the sun was obscured by cloud. It was suggested (Bauwens et al., 1990) that the high and constant body temperatures were also achieved by restricting activity only to those periods when environmental conditions made it possible to do so.
However there are high costs in respect of time budgets for reptiles in cold climates. In an analysis of the time devoted each day to the various activities of Liolaemus, Pearson & Bradford, (1976) observed that 16% of the lizard's time was spent thermoregulating (3.5 hours per day) only 0.3% (less than 5 minutes) feeding, a further 26 minutes to social behaviour and locomotory activity and for more than 80% of their time they were inactive in their burrows ( however time spent in the burrowing part at least, is really thermoregulatory behaviour, since one of the primary functions here is avoiding critically low body temperatures). Long and frequent basking periods are also part of the costs that high altitude L.bedriagae incur for precise thermoregulation in Corsica. However extensive basking in L.bedriagae results in similar body temperature levels to the closely related Podarcis tiliguerta and P.sicula at sea level where there are much higher environmental temperatures (Bauwens et al., 1990).
The costs for living in harsh environments were demonstrated by research on the viviparous lizard (Lacerta vivipara). Lacerta vivipara is found over a range of altitudes and its distribution stretches from north of the Arctic circle to the south to Spain- a distance of almost 3000 km, and east to the Pacific coast, almost 12,000 km. Within this area Lacerta vivipara populations inhabit a whole series of thermal environments and provide a useful tool to make direct intra-specific comparisons. Van Damme et.al,. (1990) compared the thermal physiology of a population at an altitude of 2000-2200m in the Austrian Alps with one at 25m in Belgium and found that body temperatures of the montane populations were consistently 3-5° below those of the lowland population. The montane lizards were often observed active at body temperatures that seriously affected locomotory capacity. There was however no parallel shift in optimal temperatures for running speeds in the high altitude lizards and complementary laboratory thermal gradient studies on individuals from both populations reinforced the findings. These observations support the static view of thermal physiology, that thermal set points are resistant to directional selection.
Van Damme et al., (1989) investigated altitude variation of the thermal biology of Podarcis tiliquerta on the island of Corsica. At high altitudes lizard body temperatures were lower and more variable than low altitude populations. The main response to changing thermal conditions was a shift to lower thermal set points but results in impaired running speeds, particularly in the early morning. P. tiliquerta has a thermal physiology that is apparently evolutionary conservative.
Thermoregulation at high latitudes
Even to the casual observer the increasing number of reptile species and their increasing abundance in terms of sheer numbers of individuals as one moves north or south towards the equator is apparent. Although the numerical gradient is not continuous -irregularities arise with local climatic and ecological variables, the general pattern of distribution appears to be consistent ( Dobzhansky, 1950).
Research into the thermal biology of (for example) both cool and warm temperate European reptiles indicate that the majority are at least partially heliothermic often living in relatively open habitats (Fig 4b) where sunshine is easily accessible and, particularly in the case of those lizard species living in the Mediterranean region, they often maintain high body temperatures of >38°C (see Spellerberg, 1976; Avery, 1982, for general reviews and Meek and Avery, 1988 for chelonians). Two species at least are believed to be thigmothermic- utilizing heat from the substrate, although one of the two, the slow-worm Anguis fragilis- has a rather complex thermal lifestyle often operating at low body temperatures but on occasion can be seen basking (Spellerberg, 1976 and pers. observation). Generally those species that live at the highest latitudes maintain the lowest activity temperatures (eg Lacerta uivipara 30-33°C; Anguis fragilis <30°C) but there is at present insufficient information to generalise usefully about latitudinal variations in body temperatures and thermoregulatory patterns. In the slow-worm it may be that much of its activity patterns are generated by the activity patterns of its prey species (slugs and earthworms). Thermoregulation may only be of secondary importance in Anguis and for much of its time it may operate as a thermoconformer.
Behavioural complexity has been shown to be inversely related to climate and latitude in certain European lacertid lizards (Avery, 1976). Thermoregulatory behaviour in the cool temperate Lacerta vivipara occupies a greater fraction of the daily time budget than in the warm temperate Podarcis (= Lacerta) sicula. The northern species consequently has less time available to evolve complex social behaviour.
Thermoregulation in Chelonians
The thermal relations of Chelonians have received less attention than those of lizards. The work carried out to date has indicated that most species regulate their body temperature by basking and retreating into shade or water. Body temperatures of those studied either in the laboratory (Hutchison, 1979) or in the field (Avery, 1982; Meek & Avery, 1982), rarely exceed 34°, the exceptions are certain semiaquatic Emydids (eg Graptemys & Trachemys(=Pseudemys)) although the desert living Gopherus maintain relatively high body temperatures (>37°C, Brattstrom, 1965).
Semi-aquatic terrapins. The evolution of the chelonian shell appeared early in the history of the group (Romer, 1968) but the shells of present day forms vary noticeably between species and such differences can significantly influence their thermal biology. Many species of semiaquatic terrapins often spend long periods basking on river banks or logs to elevate their body temperature (Boyer, 1965). Experiments with water-filled models of aquatic terrapins painted black showed that flatter bodied species had faster rates of heating but the difference could be modified by the reflectivity of the integument; therefore although the flat shaped soft shelled terrapins Trionyx had the highest rates of heat gain as far as the models were concerned, in real life darker shelled emydids of the same mass had similar rates of heat gain. Apparently the darker colour (and possibly the different texture of the emydid shell) has selective advantages for their lifestyle and cancels out any differences (Boyer 1965).
The more spherical shape retained by the emydids could also have selective advantages since it would enhance heat retention, particularly useful in the temperate zones where one of the major problems encountered by a terrapin basking on a log on a sunny Spring day could be in experiencing very cold temperatures when re-entering the water. In habitats where environmental temperatures are high, basking even in emydids may be limited (eg Meek, 1983), confined to surface water basking or be abandoned altogether (Avery, 1982 for review). Basking and precise thermoregulation may be only of minor importance (if at all) in non-emydid forms, the animals in effect operating as thermoconformers, for example the Kinosternidae (Cagle, 1944; Edgren & Edgren, 1955; reviews in Brattstrom, 1965; Avery, 1982)). However basking in terrapins is a complex behaviour and its duration in (for example) Trachemys (=Pseudemys) scripta has been shown to vary between season, sex, temperature and digestive state (Hammond et al., 1988). Female Trachemys basked longer in spring and summer than males and fed animals of both sexes basked for longer than unfed animals. In autumn and winter such differences apparently did not exist. At high environmental temperatures both sexes, whether fed or unfed, spent significantly less time basking.
Ecological influences other than thermoregulation are clearly involved in chelonian morphometry, for example predation, sexual strategy and movement mechanics have had important influences in their evolutionary biology. In aquatic forms a low profile shell design may reduce resistance to the water, but be less useful against predators and for enhancing egg production in females (Iverson, 1984; Meek, 1987). Indeed the primary function of basking although generally regarded as thermoregulatory may have secondary uses, the drying out of the shell could assist the animals in controlling parasites or reducing algal growth which can cause severe deterioration of the shell (Cagle, 1950; Neill & Allen, 1954; Meek, 1987) and may be involved in the production of vitamin D (eg Holick, 1989).
Terrestrial chelonians. The terrestrial tortoises are slow moving (Jayes & Alexander, 1980) and often grow to very large sizes which may give rise to acute problems of overheating in areas with limited shade (Swingland & Frazier, 1979) or in the smaller species restrict movement through open areas (Meek, 1984; Branch, 1984). Although the larger species, as a consequence of their low surface to body mass geometry, reduce rapid heat exchange with the environment, this can present particular problems of overheating and even heat death through thermal inertia (Swingland & Frazier, 1979). Body geometry and locomotion are clearly important constraints on chelonian thermal ecology, however predation, a major constraint on thermoregulation (Huey, 1982) is reduced as a result of the heavy armour provided by the shell.
Environmental constraints on thermoregulatory behaviour has been usefully examined in Mediterranean tortoises particularly Testudo graeca and Thermanni. The earliest works concerned T graeca populations in North Africa (Lambert, 1981, 1983; Meek & Jayes, 1982) where cloud cover (Lambert, 1981) or sea mists (Meek & Jayes, 1982) were observed to prolong basking into the latter part of the day, delaying the attainment of maximum body temperatures of 35°C to later in the day than on days when the skies were clear and sunny. In Europe T graeca were observed by Wright et al, (1988) during the summer months in Greece where they occupied relatively open habitat and attained body temperatures of 33°C or higher. These body temperatures were slightly higher than those recorded in N. Africa by Meek & Jayes, 1982 (=29.8°C) under sea mists (Greece, =30.9,29.7 & 31.1°C).
In Greece, the closely related T. hermanni was often found with T. graeca in habitats of similar structural complexity and with similar activity periods and body temperatures (Wright et al,. 1988). However T. hermanni also occupied less open habitat such as pine and broad leafed woodland, where activity shifted from the bimodal pattern observed in the open heathland areas to a unimodal pattern with significant midday activity. Body temperatures of T. hermanni were lower in enclosed habitats. This is in general agreement with body temperature levels of T. hermanni in woodland areas in southern France where there is also only limited sunlight penetration and limited basking activity (Pulford et al, 1984). Seasonal shifts in activity patterns and body temperatures have been observed in several populations of T. hermanni. Hot summer weather induced bimodal activity and high body temperatures (34-35°C) in Croatia, Montenegro and Greece ; spring and autumn produced unimodal activity and generally lower body temperatures (Meek, 1984; 1988a; Panagiota & Valakos, 1992). Panagiota & Valakos, (1992) observed winter activity at low body temperatures in T. marginate in Attica although in the same area T. hermanni entered hibernation.
The work of Wright et al,(1988) made an important contribution to the thermal biology of Mediterranean tortoises since it identified habitat use and separation and the results were discussed in the context of the group's evolutionary history. For such purposes analysis was restricted to the thermal loads of the population as a collective unit (ie the data sets from both sexes were pooled). However significant size differences exist between the sexes of T hermanni (females may be as much as twice as large as males in wild populations, eg Meek, 1985; 1989, Meek & Inskeep, 1981) and in the former Yugoslavia this was found to significantly influence thermoregulatory behaviour in different ways through different seasons. In spring T hermanni displays the classical paradigm of reptilian heliothermy with basking mainly occurring in the earlier part of the day and other activities such as feeding and locomotory activity initiated once body temperatures of 34°C or so are reached (Meek, 1984). In summer the total time spent basking is reduced allowing increased time available for feeding, locomotory and sexual activity, but females due to their larger size experience a thermal lag not attaining operative body temperatures until later in the day than males (Meek, 1988a).
How does thermal lag affect the daily lives of tortoises and indeed is it significant enough to have fundamental consequences for their evolutionary biology? In the region where these tortoises were studied , they are close to their northernmost limits in Europe and the environmental conditions found here and the thermal lag they produce on the larger species of reptile may ultimately impose maximum size constraints, since the amount of available basking time sets absolute limits on what gross body temperatures may be achieved daily and annually. Interestingly large females in this region do not increase basking intensity during cooler spring and summer weather to compensate for their larger size but compromise by accepting lower body temperatures whilst expanding feeding periods. In autumn females increase basking intensity but are still unable to achieve normal operative body temperatures - although the smaller sized males manage this (Meek, 1988a).
It would appear that T hermanni females have evolved large size to maximise egg production and operate a compromise between physiological optimum and ecologically attainable temperatures (Meek, 1988a). Male T hermanni size limitations may involve greater mobility,
for example to make contact with greater numbers of females, which would be enhanced by the Qio effects of higher body temperatures. Although thermal lag is not as significant in juvenile T hermanni thermal ecology, other factors such as predation or habitat familiarity may have an important role in restricting juvenile body temperatures to slightly lower levels than those of adult males (Meek, 1988a). Predation has been suggested as a constraint on basking in hatchling terrapins (Jansen eL al., 1992) and habitat familiarity on the body temperatures of introduced tortoises (Chelazzi & Calzolia, 1986).
The thermal environment does not rigidly constrain all reptiles to a series of homogenous responses. In former Yugoslavia where T hermanni were studied, the sympatric glass lizard (Ophisaurus apodus) is active on cool cloudy days when tortoises remain in their overnight retreats. The lizards are active at significantly lower body temperatures than is normal for their activity when they are much more at risk to attacks from predators (Meek, 1984). Hailey (I 984) has drawn attention to similarities in the physiological ecology of Ophisaurus with Testudo, in particular its reliance on dermal armour as a defence mechanism (Ophisaurus is 70% as armoured as a similar sized Testudo) and similar low metabolic scope.
Concluding remarks
The study of the thermal ecology in reptiles now encompasses such a wide range of disciplines that in this relatively short selective review it has not been possible to even briefly mention works from other major lines of investigation. References to research on, for example, environmental sex determination (review; Janzen & Paukistis, 1991), thermal constraints on nesting female reptiles (Spotila & Standora, 1985; Meek, 1988b), partial endothermy in sea turtles, monitor lizards and female pythons (review in Bartholomew 1982), the role of the parietal eye in reptile thermoregulation (Firth & Turner, 1982), thermal involvement in reproductive cycles (eg Licht, 1972 and review in Duval et al., 1982), winter dormancy (Gregory, 1982) and strategies to overcome conflicting thermal requirements of female lizards and their developing embryos (Beuchat, 1986) have been omitted although such lines of enquiry are of critical importance to our understanding of the subject. Even the medical aspects of reptilian infections, again not reviewed here, can tell us much about the evolutionary background of thermal biology. For example the way certain lizards Dipsosaurus, Agama and Sceloporus apparently respond to pathogens by inducing an "ectothermic fever" to enhance their survivorship capability is an intriguing aspect of thermal biology that may ultimately throw new light on the origins of host defence mechanisms to bacterial infections in vertebrates (Kluger, 1979; Ortega et al., 1991).
Previously reptile ectothermic physiology was regarded as an inferior physiological system in comparison to that of the endothermic mammals and birds, as if reptiles were in some way failed endotherms. Research has now shown the fallacy of this view and has indicated reptilian ectothermy as an evolutionary route in the direction of a low budget energy cost physiology which drains environmental resources at a less intense rate than mammals and birds enabling ectothermic vertebrates to colonise large areas of the world, often in high population densities.
Acknowledgement
Dr Roger Avery critically reviewed the manuscript.
References
Avery R.A. (1976). Thermoregulation, metabolism and social behaviour in Lacertidae. In Bellairs, A.d'A and Cox C.B. (eds), Morphology and Biology of Reptiles 245-259. Linnean Society Symposium Series 3. Academic Press, London.
Avery R.A. (I 982). Field studies of body temperatures and thermoregulation. In Gans, C. and Pough, F.H.(eds), Biology of the Reptilia 12, Physiology C. Physiological Ecology. 93-166. Academic Press, London.
Avery R.A. (1985). Thermoregulatory behaviour of reptiles in the field and in captivity. In Townson, S. and Lawrence, K. (Eds), Reptiles: Breeding, Behaviour and Veterinary Aspects. British Herpetological Society, London.
Avery, R.A. and Bond, D.H. (1987). Environmental constraints on lizard foraging behaviour. Applied Animal Behauioural Science 18: 348-385.
Bauwens, D., Castilla A.M., Van Damme, R. and Verheyen, R.F. (1990). Field body temperatures and thermoregulatory behaviour of the high altitude lizard Lacerta bedriagae. Journal of Herpetology 24, 88-91.
Bartholomew, G.A. (1982). Physiological control of body temperature. In Gans, C. and Pough, F.H. (Eds), Biology of the Reptilia 12, Physiology C: Physiological Ecology. 167-21 1. Academic Press, London.
Beuchat, C.A. (1986). Reproductive influences on the thermoregulatory behaviour of a live-bearing lizard. Copeia 1986, 971-979.
Berkum, F.H. (1986). Evolutionary patterns of the thermal sensitivity of sprint speed in Anolis lizards. Evolution 40, 594-604.
Boyer, D.R. (1965). Ecology of the basking habit in turtles. Ecology 46,99-118.
Branch, W.R. (1984). Preliminary observations on the ecology of the angulate tortoise (Chersina angulata) in Eastern Cape Province, South Africa. Amphibia-Reptilia 5, 43-55.
Brattstrom, B.H. (1965). Body temperatures of reptiles. American Midland Naturalist 73: 376-422
Cagle, F. R. (1944). Home range, homing behaviour and migration in turtles. Misc. Publ. Mus. Zool. Univ. Michigan. (6i), 1-34.
Cagle, F.R. (1 950). The life history of the slider turtle Pseudemys scripta troostii. Ecological Monographs 20, 31-54
Chelazzi, G. and Calzolai, R. (1986). Thermal benefits from familiarity with the environment in a reptile. Oecologia 68, 557-558.
Cowles, R.B. and Bogert, C.M. (1944). A preliminary study of the thermal requirements of desert reptiles. Bull. Am. Mus Nat. Hist. 82, 265-296.
Crowley, S.R. (1985). Thermal sensitivity in sprint-running in the lizard Sceloporus undulatus: support for a conservative view of thermal physiology. Oecologia (Berlin) 66:219-225.
Dobzhansky, T. (1950). Evolution in the tropics. Am. Scient., 38, 209221.
Duvall, D., Guillette Jr., L.J. and Jones, R.E. (1982). Environmental control of reptilian breeding cycles. In Gans, C. and Pough, F.H. (Eds) Biology of the Reptilia 13 Physiology D. Physiological Ecology 201-231. Academic Press, London.
Edgren, R.A. and Edgren, L.H. (1955). Thermoregulation in the musk turtle, Sternotherus odoratus. Herpetologica II: 213-217.
Firth, B.T. and Turner, J.S. (1982). Sensory, neural and hormonal aspects of thermoregulation. In Gans, C. and Pough, F.H. (Eds) Biology of the Reptilia 12, Physiology C. Physiological Ecology. 213-274. Academic Press, London.
Gatten, R.E. (1974). Effects of nutritional status on the preferred temperature of the turtles Pseudemys scripta and Terrapene ornata. Copeia 1974, 912-917.
Gregory, P.T. (1982). Reptilian hibernation. In Gans, C. and Pough, F.H. (Eds). Biology of the Reptilia 13 Physiology D. Physiological Ecology 53-154. Academic Press, London.
Hailey, A. (1982). Choice of substrate and heating rate in Lacerta vivipara. British Journal of Herpetology 6, 207-213.
Hai I ey, A. (1984). Thermoregulation and activity metabolism in the armoured anguid Ophisaurus apodus. British Journal of Herpetology 6, 391-398.
Hammond, K.A., Spotila, J.R. and Standora, E.A. (1988). Basking behaviour of the turtle Pseudemys scripta: effects of digestive state, acclimation temperature, sex and season. Physiological Zoology 61, 6977.
Hertz, P.E., Huey, R.B. and Nevo, E. (1983). Homage to Santa Anita: thermal sensitivity of sprint speed in agamid lizards. Euolution 37:10751084.
Hertz, P.E. (1992). Temperature regulation in Puerto Rican Anotis lizards: a field test using null hypotheses. Ecology 73, 1405-1417.
Heath, J.E. (1964). Reptilian thermoregulation: evaluation of field studies. Science, N.Y. 145, 748-785.
Holick, M.F. (1989). Phylogenetic and evolutionary aspects of vitamin D from photoplankton to humans. In Vertebrate Endocrinology: Fundamentals and Medical Implications. 7-43, vol 3 (Eds. Pang, P.K.T. and Schreibman, M.P.) Academic Press, Orlando, Florida.
Huey, R.B. (1982). Temperature, physiology and the ecology of reptiles. in Gans, C. and Pough, F.H. (Eds.), Biology of the Reptilia 12 Physiology C: Physiological Ecology. 25-9 1. Academic Press, London.
Huey, and Slatkin, M. (1976). Costs and benefits of lizard thermoregulation. Quarterly Review of Biology 51: 363-384.
Huey, R.B. and Bennett, A.F. (1987). Phylogenetic studies of coadaptation: preferred temperatures versus optimal performance temperatures of lizards. Evolution 41:1098-1115.
Hutchison, V.H., Vinegar,A and Kosh, R.J. (1966). Critical thermal maxima in turtles. Herpetologica 22, 32-41.
Hutchison, V.H. (1979). Thermoregulation. In Hariess, M. and Morlock, H.(Eds.) Turtles: Perspectives and Research. 207-228. Wiley.
Iverson, J.B. (1984) Proportional skeletal mass in turtles. Florida Scientist, 47, 1 -1 1.
Janzen, F.J., Paukstis, G.L. and Brodie, E.D. (1992). Observation on basking behaviour of hatchling turtles in the wild. Journal of Herpetology 26, 217-219.
Jayes, A.S. and Alexander R. McN (1980). The gaits of chelonians: walking techniques for very low speeds. Journal of Zoology. London. 191, 353-378.
Kluger, M.J. (1979). Fever in ectotherms: evolutionary implications. American Zoologist 19, 295-304.
Lambert, M.R.K. (1981). Temperature, activity and field sighting in the Mediterranean spur-thighed or common garden tortoise Testudo graeca. Biological Conservation 21, 39-54.
Lambert, M.R.K. (1983). Some factors influencing the Moroccan distribution of the western Mediterranean spur-thighed tortoise Testudo graeca, and those precluding its survival in N.W. Europe. Zoological Journal of the Linnean Society 79, 149-179.
Licht, P. (1972). Environmental physiology of reptilian breeding cycles; role of temperature. General and Comparative Endocrinology Supplement 3, 477 -488.
Meek, R. (1978). On the thermal relations of two oriental Varanids: Varanus bengalensis nebulosis and Varanus salvator. Cotswold Herpetological Symposium Report 1978, 32-47.
Meek, R. (1983). Body temperatures of a desert population of the stripe-necked terrapin, Mauremys caspica. British Journal of Herpetology 6, 335-337.
Meek, R. (1984). Thermoregulatory behaviour in a population of Hermann's tortoise (Testudo hermanni) in southern Yugoslavia. British Journal of Herpetology 6, 387 -39 1.
Meek, R. (1985). Aspects of the ecology of Testudo hermanni in southern Yugoslavia. British Journal of Herpetology 6, 437-445.
Meek, R. (1986). Field body temperature of the glass lizard Ophisaurus apodus in Yugoslavia. Amphibia-Reptilia 7, 43-49.
Meek, R. (1987). Aspects of the population ecology of Mauremys caspica in North West Africa. Herpetological Journal 1, 130-136.
Meek, R. (1988a). The thermal ecology of Hermann's tortoise (Testudo hermanni) in summer and autumn in Yugoslavia. Journal of Zoology, London 215, 99-1 1 1.
Meek, R. (1988b). Thermal loads experienced by a nesting female Testudo hermanni. Amphibia-Reptilia 9, 311-312.
Meek, R. (1989). The comparative population ecology of Hermanns tortoise, Testudo hermanni in Croatia and Montenegro, Yugoslavia. Herpetological Journal 1, 404-414.
Meek, R. and Inskeep, R. (1981). Aspects of the field biology of a population of Hermanns tortoise (Testudo hermanni) in southern Yugoslavia. British Journal of Herpetology 6, 159-164.
Meek, R. and Jayes, A.S. (1982). Body temperatures and activity patterns of Testudo graeca in North West Africa. British Journal of Herpetology 6, 194-197.
Meek, R. and Avery, R.A. (1988). Thermoregulation in chelonians. Herpetological Journal 1,253-259.
Neill, W.T. and Allen E.R. (1954). Algae on turtles: some additional considerations. Ecology 35, 581-584.
Obbard,M.E. and Brooks,R.J. (1979). Factors affecting basking in a northern population of the common snapping turtle, Chelydra serpentine. Canadian Journal of Zoology 57, 435-440.
Ortega, C.E., Stranc, D.S., Casal, M.P., Hallman, G.M. and Muchlinski, A.E. (1991). A positive fever response in Agama agama and Sceloporus orcutti. Journal of Comparative Physiology B: 161, (4) 377381.
Panagiota, M. and Valakos, D. (1992). A contribution to the thermal ecology of Testudo marginate and T hermanni in semi-captivity. Herpetological Journal 2, 48-50.
Pearson, O.P. (1954). Habits of the lizard Liolaemus m. multiformis at high altitudes in southern Peru. Copeia 1954, 111-116.
Pearson, O.P. and Bradford, D.F. (1976). Thermoregulation of lizards and toads at high altitudes in Peru. Copeia 1976, 155-170.
Pulford, E., Hailey, A. and Stubbs, D. (1984). Thermal relations of Testudo hermanni robertmertensi in S. France. Amphibia-Reptilia 5, 37-41.
Romer, A.S. (1968). Osteology of The Reptiles. The University of Chicago Press, Chicago.
Sergeyev, A.M. (1939). The body temperatures of reptiles in natural surroundings. Comptes Rendus de LAcademie des Sciences de l'URSS. 22, 49-52
Spellerberg, I.F. (1976). Adaptations of reptiles to cold. /n Bellairs, A-d'A. and Cox, C.B. (Eds.) The Morphology and Biology of Reptiles, 261-285. Linnean Society Symposium Series 3. Academic Press, London.
Spotila, J.R. and Standora E.A. (1985). Environmental constraints on the thermal energetics of sea turtles. Copeia 1985, 694-702.
Strelnikov, S.D. (1934). Light as a factor in animal ecology. 1. The influence of solar radiation on the body temperatures of some poikilothermic animals. Izu. Nauchn. Inst. Im. & Zool. Lesgafta 17-18, 313-372. (in Russian).
Streinikov, S.D. (1944). Importance of solar radiation in the ecology of high mountain reptiles. Zool. Zh. 23:250-257.
Sturbaum, B.A. (1982). Temperature regulation in turtles. Comparative Biochemistry and Physiology 72A, 615-620
Swingland, I.R. and Frazier J.G. (1979) The conflict between feeding and overheating in the Aidabran giant tortoise. In Amlaner, C.J. and MacDonald, D.W. (Eds.) A handbook of biotelemetry and radio tracking. 611-615. Pergamon Press, Oxford.
Turner, F.B., Medica,P.A. and Kowalewsky, B.W. (1976). Energy utilisation by a desert lizard (Uta stansburiana). US-IBP Desert Biome Monograph No 1, Utah State University Press. Logan.
Van Damme, R., Bauwens, D., Castilla, A.M. and Verheyen, R.F. (1989). Altitudinal variation of the thermal biology and running performance in the lizard Podarcis tiliquerta. Oecologia (1989) 80:516-524.
Van Damme, R., Bauwens, D. and Verheyen, R.F. (1990). Evolutionary rigidity of thermal physiology: the case of the cool temperate lizard Lacerta viuipara. Oikos 57: 61-67. Copenhagen 1990.
Wright, J., Steer, E. and Hailey, A. (1988). Habitat separation in tortoises and the consequences for activity and thermoregulation. Canadian Journal of Zoology 66, 1537-1544.
Testudo Volume Four Number Two 1995
Top