S. Nathanael¹ S.M.M. Samarakoon¹ N. D. Warusamanna¹ R.P.V.J. Rajapakse² and Anslem de Silva³
¹ Department of Biology, Faculty of Applied Sciences, Rajarata University of Sri Lanka, Polgolla, Sri Lanka.
² Department of Veterinary Pathobiology,Faculty of Veterinary Medicine and Animal Science, University of Peradeniya, Peradeniya, Sri Lanka. (E- mail :jayanthar{at}dn.ac.lk)
³15/1, Dolosbage Road, Gampola, Sri Lanka (E-mail: kalds{at}sltnet.lk)
Introduction
The Indian star tortoise Geochelone elegans (Schoepff, 1795) is a beautiful land tortoise which gets its name from the radiating star pattern on its shell. It is the only representative of the family Testudinidae in Sri Lanka. Wild populations are confined mainly to the lowlands in the dry zone of the country. The star tortoise is a vulnerable species currently protected in Sri Lanka under the Fauna and Flora Protection Act of 1972 and the 7th amendment to the Act in 1993 and a CITES schedule II listed animal.
It is documented that both internal and external parasitic infestations are major health hazards for tortoises (Silva et al, 1998). One of the earliest reports of parasitic infestation in Sri Lankan tortoises was by Deraniyagala (1939). More recent observations (Rajapakse et al, 1998) in Sri Lanka reveal that most wild tortoises are heavily infested externally with hard ticks and internally with gastrointestinal nematodes. No such information is available hitherto, for tortoises kept in captivity. The present survey was undertaken, therefore, with the objective of assessing the status of captive Geochelone elegans in relation to its vulnerability to parasites and what management measures the pet owner should adopt.
Methods
A total of 54 star tortoises kept as pets by 19 owners in different parts of the country were investigated during this study conducted from April to September 2003.
The star tortoises were thoroughly checked for ectoparasites. These were removed using forceps, stored in 70% alcohol, transported to the laboratory and identified according to the key described by Seneviratna (1965). To check for gastrointestinal parasites, 30 faecal samples were collected from different tortoises in captivity, into small vials containing 10% formalin and analysed in the laboratory by making smears and by using a simple salt flotation method (Rajapakse et al, 1998). During the survey a structured questionnaire was used to collect morphometric measurements of the tortoises as well as important information relevant to the transmission of parasites such as the type of food given, the presence or absence of grass in the environment, time period in captivity, and housing and care.
Parasites of captive tortoises
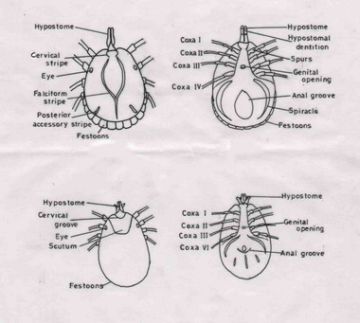
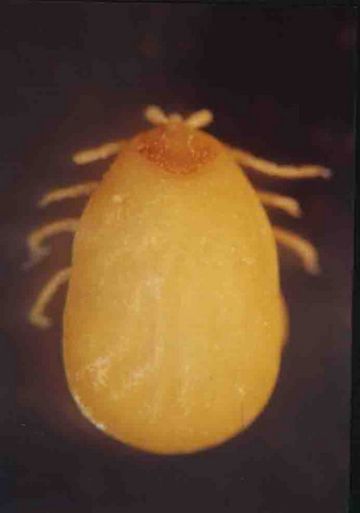
Of the star tortoises examined during the survey, 18 were males, 33 were females and 3 were not sexed. Of these 54 tortoises, mild infestation with two species of ectoparasitic hard ticks, namely, Amblyomma clypeolatum and A. testudinarum (Fig.1), belonging to the family Ixodidae, was observed between the carapace and forelegs of only two (3.7%) tortoises. In contrast, it is recorded that 88% of the tortoises examined in the wild showed severe infestation with the same two species of tick (Rajapakse et al, 1998). Figs.3, 4 and 5 show how these ticks match the colouring of the carapace.
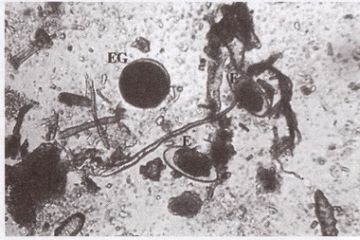
Analysis of faecal samples revealed that 46.7% of the tortoises (Table 1) were infected with three different types of nematode eggs, which could be differentiated by their morphology. Of these, the ascaroid and oxyuroid type eggs (Fig.2) were the most common. The egg count per gram of faeces (EPG) of captive tortoises varied from 2 to 105 eggs with minimum infection with one type of gastrointestinal nematode. In contrast, it is recorded that 67.5% to 99% of tortoises in the wild were infected with gastrointestinal nematodes (de Silva, 1995; Rajapakse et al, 1998), and egg counts were very high, ranging from 400 to 12,000 eggs per gram of faeces (Rajapakse et al, 1998).
| No. | House no. | Sex | Curved Carapace Length (mm) | Body Weight (kg) | Time period in Captivity | Feeding Habit | Grass | Intensity of Infection |
|---|
| 1 | 1 | F | 307 | 2.250 | 10 months | L,V | P | NI |
| 2 | 1 | M | 197 | 0.250 | 2 months | L,V | P | NI |
| 3 | 1 | F | 140 | 0.207 | 1 month | L,V | P | ML |
| 4 | 1 | F | 99 | 0.093 | 5 months | L,V | P | ML |
| 5 | 2 | M | 242 | 1.050 | > 20 years | L,V | A | NI |
| 6 | 3 | F | 350 | 3.000 | > 2 years | L,F | A | NI |
| 7 | 4 | F | 368 | 3.200 | > 1 year | G,V,L | P | NI |
| 8 | 4 | F | 370 | 4.000 | > 1 year | G,V,L | P | NL |
| 9 | 5 | M | 261 | 0.950 | > 10 years | V,F,B | P | ML |
| 10 | 5 | F | 346 | 3.200 | > 10 years | V,F,B | P | ML |
| 11 | 6 | F | 298 | 2.350 | > 3 years | V,F | A | NI |
| 12 | 7 | M | 271 | 1.700 | > 4 years | V,G | P | NI |
| 13 | 7 | M | 226 | 0.900 | 2 years | V,G | P | NI |
| 14 | 7 | F | 325 | 2.500 | > 4 years | V,G | P | ML |
| 15 | 7 | F | 317 | 2.700 | > 7 years | V,G | P | NI |
| 16 | 7 | F | 336 | 2.950 | > 4 years | V,G | P | NI |
| 17 | 8 | M | 370 | 2.000 | > 1½ years | B,V,F | A | NI |
| 18 | 8 | F | 320 | 2.100 | > 1½ years | B,V,F | A | NI |
| 19 | 8 | F | 300 | 1.950 | > 1½ years | B,V,F | A | NI |
| 20 | 9 | F | 315 | 2.400 | > 3½ years | V,F | P | NI |
| 21 | 9 | F | 313 | 2.400 | > 3½ years | V,F | P | ML |
| 22 | 9 | F | 311 | 2.500 | > 3½ years | V,F | P | ML |
| 23 | 9 | F | 291 | 2.200 | > 3½ years | V,F | P | MO |
| 24 | 9 | F | 358 | 3.000 | > 3½ years | V,F | P | MO |
| 25 | 9 | M | 283 | 1.440 | > 3½ years | V,F | P | MO |
| 26 | 10 | M | 264 | 1.000 | > 1 year | L,V | A | NI |
| 27 | 11 | M | 241 | 0.950 | > 20 years | R,V,F | A | ML |
| 28 | 11 | F | 367 | 4.000 | > 20 years | R,V,F | A | MO |
| 29 | 12 | F | 353 | 3.500 | > 5 years | G,V,F | P | ML |
| 30 | 12 | F | 323 | 2.900 | > 5 years | G,V,F | P | NI |
| (Abbreviations: NI Not Infected; ML mild > 50 eggs per gram of faeces; MO moderate 50-200 eggs per gram of faeces; M=Male; F=Female; L=Leaves; V=Vegetable; G=Grass; B=Bread; R=Rice; F=Fruits;P=Present; A=Absent). |
Table 1: Morphometry, food, time period in captivity etc. and state of gastrointestinal nematode infection of the captive star tortoises.
Transmission of parasites
One of the reasons for the scarcity of Amblyomma ticks on captive tortoises could be that in animals housed singly, cross infection cannot occur. Young ticks are very vulnerable to dehydration and might not be able to survive in many captive environments. Also, of course, many owners remove ticks from their pets.
According to the results obtained in this survey, transmission of gastrointestinal nematodes appears to occur irrespective of the time period in captivity, body size or sex of the tortoise (Table 1).
The oral route is the main mode for transmission of most gastrointestinal parasites. Thus, food is an important means by which tortoises could become infected with parasites, their eggs or larvae. The tortoises surveyed were maintained on a diet consisting predominantly of fresh leaves, vegetables and fruits which were either garden produce or purchased from a nearby market. Hibiscus flowers and leaves, grasses, vegetables such as lettuce, pumpkin, cabbage, beans, potato, carrot, snake gourd and cucumber, and fruits such as papaya, water melon, bananas, avocado and mango were some common food items consumed. However, it was not possible to relate the worm burden of the tortoise to its feeding habit. Hence, more detailed studies are required on this aspect.
Apart from food, housing and care are crucial factors for transmission of parasites to tortoises in captivity, since the stress of overcrowding, improper attention, lack of proper nutrition and being with mixed species, or the same species which are infected with parasites, inevitably make the tortoise susceptible to infection.
The tortoises observed during the present survey had been kept as pets for periods ranging from one month to over 20 years. One tortoise was reared together with two giant squirrels, while the other tortoises were maintained either singly or as an isolated group. The tortoises were kept outdoors, in cages, pens and enclosures with or without grass, measuring a minimum of 2 x 1 metres. Since tortoises feed on grass, it is possible that grass acts as an important means for transmission of parasites, their eggs or larvae. In the present study 57% of captive tortoises from habitats where grass was present were infected with gastrointestinal nematode eggs. Where grass was not present only 22% were infected. Oxyuroid eggs and larvae in particular are much more likely to survive when grass is present.
Recommendations for pet owners
The findings of this study indicate that star tortoises in captivity are subject to parasitic infestations. In order to maintain the tortoises in good health it is necessary to educate tortoise keepers on treatment of parasites. Ectoparasites such as ticks could be treated with an acaricide such as Coumaphos (Asuntol), Amitraz (Tactic; RIDD), Flumethrin (Bayticol) and Propoxur (Bolfo) which are freely available for small animal practices in Sri Lanka*, while commonly used antihelminthic drugs such as Pyrantol Parmoate (Combantrin) and Mebandazole (Vermox) could be used for gastrointestinal nematodes. However, further studies are needed to formulate correct dosages for tortoises. Since food is an important means through which parasite eggs and larvae could be transmitted precautions need to be taken by washing vegetables, leaves, etc. thoroughly before feeding tortoises, particularly foods which come from an endemic area.
Acknowlegements
Thanks are due to Dr (Mrs) Manel Goonesekera, Head of Department of Biology, for provision of facilities to carry out this survey. A special word of thanks to the British Chelonia Group and to Professor Aaron Bauer (University of Villanova, USA) who financially supported the students of this university to work on captive tortoises through a seed grant to Anslem de Silva. We also wish to thank Mr Tissa Alagoda (Department of Zoology, University of Peradeniya) for assistance with one of the illustrations, Mr K.N.K. Kumara for field assistance, and Ms Kanchana Thibbotuwawa and Kanthi Manathunge for assistance with the laboratory analysis.
References
Deraniyagala, P.E.P (1939). The Tetrapod Reptilia of Ceylon, Vol.1, Colombo Museum.xxxii.
de Silva, A. (1995). The status of Geochelone elegans in north western province of Sri Lanka: preliminary findings. In: Proceedings of the International Congress of Chelonian Conservation (ed. B. Devaux), France pp. 47-49.
de Silva, A. (1998). Country Report for Sri Lanka. Herpetofauna of Sri Lanka: Present status, Distribution, and Conservation. In: Biology and Conservation of the Amphibians, Reptiles and their habitats in South Asia. Proceedings of the International Conference on the Biology and Conservation of Amphibians and Reptiles of South Asia, Sri Lanka (ed. A. de Silva), ARROS pp. 51-73.
Rajapakse, R.P.V.J., Kuruwita, V.Y. and de Silva, A. (1998). Preliminary study on external and internal parasites in Geochelone elegans (star tortoise) in Sri Lanka. In: Biology and Conservation of the Amphibians, Reptiles and their habitats in South Asia. Proceedings of the International Conference on the Biology and Conservation of Amphibians and Reptiles of South Asia, Sri Lanka (ed. A. de Silva), ARROS pp. 343-349.
Seneviratna, P. (1965). The Ixodoidea (Ticks) of Ceylon. Parts II & III. Ceylon Veterinary Journal 13: (2) 28-54.
Silva, I., Mallawa M.R.C.K., Rajapakse, R.P.V.J., Kuruwita, V.Y. and de Silva, A. (1998). Blood parameters of star tortoises (Geochelone elegans). In: Biology and Conservation of the Amphibians, Reptiles and their habitats in South Asia. Proceedings of the International Conference on the Biology and Conservation of Amphibians and Reptiles of South Asia, Sri Lanka (ed. A. de Silva), ARROS pp. 355-359.
*Editor's note: drugs available in the UK will vary from those described here.
Testudo Volume Six Number One 2004
Top