James C. Gumbs
Department of Fisheries and Marine Resources, Government of Anguilla, P O Box 60, The Valley, Anguilla, West Indies
James.Gumbs{at}gov.ai
INTRODUCTION
Sea turtles spend the majority of their lives in water. Documenting the life history of these organisms is therefore difficult, and so research on foraging sea turtles is less comprehensive than that of their nesting behaviour on land (Bjorndal 1999; Epperly 2000). In order to have a more complete understanding of sea turtle ecology, more in-water research is necessary. Indeed, with regards to foraging sea turtle populations within Anguilla’s Exclusive Fishery Zone, only three studies (two general and one more extensive) have been widely published: Carr et al. (1982), Meylan (1983), and Godley et al. (2004), respectively. This study therefore aims to contribute to the existing literature on foraging sea turtles in Anguilla, while at the same time addressing our current imbalance of knowledge concerning sea turtle ecology.
Anguilla, the most northerly of the Leeward Islands in the Lesser Antilles (Fig.1), is a small coralline island (91 km2) surrounded by an array of seagrass beds, as well as by a combination of fringing, patch, and barrier coral reefs: key habitats for some species of both juvenile and adult sea turtles. Of the six species of sea turtles known to the Wider Caribbean Region, only two are often encountered around the coastal waters of Anguilla: the hawksbill turtle (Eretmochelys imbricata) and the green turtle (Chelonia mydas). A third species, the loggerhead (Caretta caretta), also occurs around Anguilla but in much smaller numbers [than the other two] and is only occasionally seen foraging in the area (Meylan 1983; Godley et al. 2004; Eckert & Hodge [in review]).
Historically, foraging sea turtle populations were abundant in Anguilla (Meylan 1983; Eckert & Hodge [in review]). Unfortunately, after decades of over-exploitation for their meat, oil, and shells, as well as habitat degradation from coastal development and hurricane activity, their numbers have dwindled. Indeed, foraging sea turtle populations in Anguilla are now only a fraction of former levels. This realization has prompted the need for an assessment of Anguilla’s foraging sea turtle populations. This study represents Anguilla’s first systematic assessment of these populations and provides invaluable data on which future in-water research can be based.
Study Sites
Turtle sampling was predominantly conducted at three sites around Anguilla’s coastline as well as at one offshore cay (Scrub Island). These sites were selected because large numbers of foraging green and hawksbill sea turtles had been observed there by informed fishers, in recent times. It should be noted that two other sites (not shown in Fig. 1) were also sampled on one occasion each during the study. The data collected from these, however, were used sparingly. Thus limited mention of these sites is made in this paper.
- Fish Hole Pond is a small pond [~ 4 hectares (ha)] located on the northeast end of Scrub Island (Fig. 1). The pond is connected to the sea via crevices in the rocks separating the two water bodies, more pronouncedly during high tide. The habitat type (bottom structure) of the pond comprises sand, seagrass beds, algae, and traces of algae and seagrass. The pond is less than 3 metres deep and is home to several species of fish, eels, stingrays, and green sea turtles.
- Scilly Cay is a small offshore cay (<1 ha) located about 200 metres from the mainland at Island Harbour, situated on the northeast end of the Island. Sampling was conducted southwest of Scilly Cay on an extensive bed of seagrass in water less than 3 metres deep.
- Little Bay/Crocus Bay is an area of coastline less than 1000 metres in length and is characterised by fringing and patch reefs in depths of less than 5 metres of water.
- North Cliffs is an area of coastline less than 600 metres in length, and is characterised by a shallow reef area partly formed of dead Acropora palmata. Water depth at this site is generally less than 2 metres.
METHODS
Sea turtles in this study were captured on a sporadic basis between September 2002 and July 2004, using the following methods:
- Hand capture: foraging hawksbill turtles captured manually using snorkelling gear and a small Whaler (boat), when not snorkelling from shore.
- Turtle net: a traditional Anguillian method of capturing sea turtles, which involves the use of a 30-cm square mesh floating net approximately 4 metres deep and 20 metres long. It is set in a given location and left in place overnight (usually 12 hours). The net is set at night and checked once in the morning. The floating net is attached to an anchor at one end via a rope, with enough scope for entangled turtles to breathe at the surface; hence turtles are not killed by drowning using this method.
- Net: a 10-cm mesh net approximately 100 metres long and 3 metres deep is deployed from a boat encircling a specific area. The perimeter of the net is then snorkelled by swimmers who catch turtles when they become entangled in the net. This method is a modification of the turtle capturing method employed by The Bermuda Turtle Project (see Meylan & Meylan 1999).
- Seine net: a seine net is extended from one end of the survey site to the other and is then hauled to the shallow area, thereby allowing turtles to be removed from the net. This method was employed at a single site, Fish Hole Pond.
Using a 150-cm fibreglass tape measure (accurate to 0.1cm), biometric measurements such as curved carapace length (CCL), straight plastron length, and head width were taken for all sea turtles captured. However, for the purpose of this paper, only the CCL data were used. In addition, skin biopsies were taken from a hind flipper of all turtles for genetic sampling (results to be presented elsewhere). Captured turtles were tagged with metal Inconel tags at the trailing edge of both front flippers and passive integrated transponder (PIT) tags in the left shoulder muscles, in accordance with standard tagging procedures (see Balazs 1999). Following tagging and data recording, turtles were released at the site of capture.
Data analysis
The average curved carapace length (CCL) in centimetres (cm) was calculated and length frequency distribution was compiled for the total number of turtles captured at each location, as well as for the total number of each species captured in the study. The mean catch per unit effort (CPUE) and the standard deviation (SD) were calculated for turtles caught at Scilly Cay, Little Bay/Crocus Bay, and North Cliffs. For captured green turtles, CPUE represented the number of turtles caught per hour of time the net was left in place, while for hawksbills it indicated the number of turtles captured per hour of time spent snorkelling.
RESULTS
The average CCL of the total number of green turtles captured during the study was 47.3cm (n=75) with individual green turtle captures ranging from 23.3cm to 80.6cm CCL (Table 1). The majority of green turtles captured were between 33.5cm and 61.4cm CCL (Fig. 2). The average CCL of green turtles captured at Scilly Cay was slightly higher than that of those captured in Fish Hole Pond (Table 1).
| Green Turtles |
|---|
| Location | Capture Method | Total Captured | Average CCL (cm) | Minimum CCL (cm) | Maximum CCL (cm) |
|---|
| Fish Hole Pond | Net | 15 | 45.3 | 32.9 | 59.5 |
| Scilly Cay | Net | 60 | 47.8 | 23.3 | 80.6 |
| Sites combined | | 75 | 47.3 | 23.3 | 80.6 |
| Hawksbill Turtles |
|---|
| Little Bay/Crocus Bay | Hand | 13 | 29.6 | 25.4 | 37.3 |
| Junks Hole Bay | Hand | 1 | - | - | - |
| Shoal Bay East | Hand | 1 | - | - | - |
| North Cliffs | Hand | 9 | 25.8 | 22.7 | 32.3 |
| Sites combined | | 24 | 27.9 | 22.7 | 37.3 |
Table 1. Data summary of turtles captured during the study
Hawksbill turtles captured during the study ranged from 22.7cm to 37.3cm CCL, with an average CCL of 27.9cm (n=24) (Table 1). The majority of hawksbills captured ranged between 25.5cm and 33.4cm CCL (Fig. 3). Hawksbill turtles captured at Little Bay/Crocus Bay were slightly larger (in terms of CCL) than those captured at North Cliffs (Table 1).
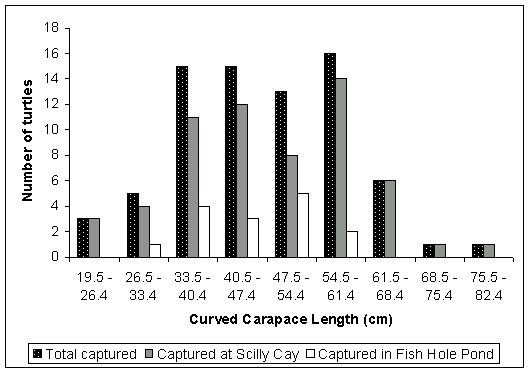
Figure 2. Length Frequency Distribution of green turtles captured in the survey.
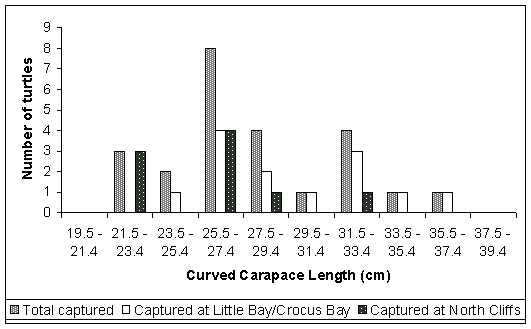
Figure 3. Length Distribution Frequency of hawksbill turtles captured in the survey.
The mean CPUE of green turtles captured at Scilly Cay using the net method was 11.4 (SD±6.4, n=5) (turtles caught per hour of time the net is left in place), while green turtles captured using the traditional turtle nets showed a mean CPUE of 1 (SD±0, n=2) (turtles caught per 12 hours of time the net is left in place). The mean CPUE of hawksbills captured at Little Bay/Crocus Bay and North Cliffs were 1.9 (SD±1.2, n=7) and 4.5 (SD±0.7, n=2) (turtles caught per hour of snorkelling time), respectively (Table 2). It should be noted here that the CPUE data are not quantitatively comparable due to the different capture methods employed as well as the differences in the habitat type of the study sites.
| Study Site | Species | Capture Method | Mean CPUE | SD | N | Min | Max |
|---|
| Scilly Cay - I. Harbour | Green | Turtle Net | 1 | 0 | 2 | 1 | 1 |
| Scilly Cay - I. Harbour | Green | Net | 11.4 | 6.4 | 5 | 2 | 20 |
| Little Bay/Crocus Bay | Hawksbill | Hand | 1.9 | 1.2 | 7 | 1 | 4 |
| North Cliffs - Katouche | Hawksbill | Hand | 4.5 | 0.7 | 2 | 4 | 5 |
Table 2. Mean CPUE and associated statistical parameters for turtles captured at 3 sites
Tables 1 and 2 show data for turtles captured and tagged for the first time only; however, 15 tagged turtles were recaptured during the study period. Of these 15 tagged turtles, 13 were recaptured at Scilly Cay, while 2 were recaptured in Fish Hole Pond. There were no hawksbill turtles recaptured during the study.
DISCUSSION
For the first time, foraging sea turtle populations of Anguilla have been studied in detail: a total of 75 green and 24 hawksbill turtles were captured and released during the September 2002 to July 2004 survey period. This preliminary study, therefore, provides baseline data for captured green and hawksbill sea turtles in their development habitats in the coastal waters of Anguilla.
Some studies (Musick & Limpus 1997; Bjorndal 1997) have shown that green and hawksbill turtles in the Caribbean recruit to near shore development habitats at approximately 20 to 25cm carapace length. This corresponds favourably with the smaller sizes of both species captured in this study. The maximum size of 80.6cm and 37.3cm CCL for green and hawksbill turtles captured in this study respectively is an indication that only juvenile turtles were captured, as adult (sexually matured) green turtles generally measure from 95 to 120cm in carapace length (Lagueux 2001), while adult hawksbills generally measure from 85 to 90cm in carapace length (Amorocho 2001).
Green turtles captured at Scilly Cay recorded a slightly higher average CCL (47.8cm, n=60) than that of those captured in Fish Hole Pond (45.3cm, n=15). This is a reflection of the fact that a higher number of the larger green turtles (CCL >54.5cm) were captured at Scilly Cay. The higher number of larger green turtles recorded at Scilly Cay is not surprising. First of all, many more turtles were captured at Scilly Cay than in Fish Hole Pond; and secondly the perceived behaviour of the larger sea turtles in Fish Hole Pond may have accounted for the smaller number of the larger green turtles being captured there.
From the account of informed observers of green turtles in Fish Hole Pond, it is assumed that during high tide in the early morning the larger size greens leave Fish Hole Pond to go foraging in the waters around Scrub Island and then return in the evening [J. Lake and P. Webster – Fishers pers. comm. May 2003]. This is logical seeing that Fish Hole Pond is a small area with a limited food supply, when compared with the waters around Scrub Island. Therefore, it is possible that during the sampling at Fish Hole Pond, which usually began at around 10 am, the larger size green turtles were not present and thus were not represented in the catches. Thus far, none of the turtles captured and tagged in Fish Hole Pond has been recaptured other than in the pond, substantiating this assumption.
The shallow inshore reef areas snorkelled in this study and the tendency for the smaller size hawksbill turtles to avoid predators such as sharks by inhabiting these reef areas (Musick & Limpus 1997) may have contributed to the relatively low average size of captured hawksbills (27.9cm CCL). Furthermore, the difference in size between the hawksbills captured at North Cliffs and those captured at Little Bay/Crocus Bay may be the result of the contrast in the behaviour of hawksbill turtles at both sites as well as the distinctions in water depths and reef morphology between the sites. That is, the hawksbills at North Cliffs were captured most of time resting under a reef ledge in shallow water, while the hawksbills at Little Bay/Crocus Bay swam around or rested next to patch reefs in deeper water. This may also explain the difference in mean CPUE recorded between the two sites since motionless turtles in shallower reef areas are easier to catch than turtles in deeper, more open water.
Although as noted earlier the mean CPUE data are not comparable because of differences in methods, at least in terms of the number of turtles likely to be caught during sampling, the net method used to capture sea turtles is more effective than the traditional turtle net method. According to an experienced former Anguillian turtle fisher (personal communication) the mean CPUE of 1 (turtle per 12 hours of time the net is left in place) recorded in this study using the traditional turtle net is the best expected given the length of net used (20 metres). Indeed, a catch of three or four turtles using a larger turtle net (50 – 75 metres) would be considered exceptional [KJ Gumbs pers. comm. March 2005]. This figure implies that it would be more feasible to employ the net method in future surveys, as it is likely that more turtles would be captured during sampling. The net method and expected results would complement one of the key in-water research objectives: to catch and tag as many turtles as possible.
This preliminary assessment did not only provide baseline data for foraging green and hawksbill turtles in Anguillian waters, it also examined the efficacy and efficiency of four different turtle capturing methods. The study showed that, for at least green turtles, it was more feasible to utilize the net method, in terms of the number of turtles likely to be caught per set. Moreover, the capture method employed with the traditional turtle net (in terms of setting the net overnight) also has significant indirect implications, in that both the nets and any turtles entangled in them are vulnerable to vandalism and thievery.
Future in-water studies in Anguilla should include the sampling of more sites around mainland Anguilla and of the offshore cays. Surveys should be conducted on a monthly basis and hawksbill sightings during surveys should be included in mean CPUE calculations. That is, based on the current method not all hawksbills spotted during the surveys are captured. If mean CPUE data are going to be used to show trends in hawksbill population abundance, data on hawksbill sightings should also be integrated into the analysis so as to provide a more accurate account of population size and health. Though no loggerhead turtles were captured during this survey, the tagging of two loggerheads brought to the Department of Fisheries (DoF) by fishers on different occasions during the sampling period is confirmation that loggerheads do occur in Anguillian waters albeit in small numbers. Thus, future work should record the areas of loggerhead sea turtle sightings as well as incidental captures within Anguillian waters with the aim of including these sites in future monitoring and assessment surveys.
ACKNOWLEDGEMENTS
I am grateful to Peter Richardson and Sue Ranger both of the Marine Conservation Society (MCS)-UK and the Turtles in the Caribbean Overseas Territories (TCOT) project for providing most of the data on hawksbill captures; and for their influential role in the in-water work on a whole. I thank the staff at the Department of Fisheries (DoF), as well as the Island Harbour fishers and other volunteers who participated in the in-water work. Many thanks to the British Chelonia Group for providing a grant for the construction of the traditional turtle nets used in this study; as well as to the Wider Caribbean Sea Turtle Conservation Network (WIDECAST), and the TCOT project for providing overseas training to three DoF staff members in in-water turtle research. A great deal of gratitude is owed to the anonymous reviewer of the manuscript.
REFERENCES
Amorocho, D.F. (2001). Status and Distribution of the Hawksbill Turtle, Eretmochelys imbricata, in the Wider Caribbean Region. In: Eckert, K.L. & Abreu-Grobois, F.A. (eds). Proceedings of the Regional Meeting: "Marine Turtle Conservation in the Wider Caribbean Region: A Dialogue for Effective Regional Management", Santo Domingo, 16-18 November 1999. WIDECAST, IUCN-MTSG, WWF, and UNEP-CEP. pp 41-45.
Balazs, G.H. (1999). Factors to consider in the tagging of sea turtles. In: Eckert, K.L., Bjorndal, K.A., Abreu-Grobois, F.A., & Donnelly, M. (eds). Research and Management Techniques for the Conservation of Sea Turtles. IUCN/SSC Marine Turtle Specialist Group Publication No. 4, 1999. pp 101-109.
Bjorndal, K.A. (1997). Foraging Ecology and Nutrition of Sea Turtles. In: Lutz, P.L. & Musick, J.A. (eds). The Biology of Sea Turtles. CRC Press, Boca Raton. pp 199-231.
Bjorndal, K.A. (1999). Priorities for Research in Foraging Habitats. In: Eckert, K.L., Bjorndal, K.A., Abreu-Grobois, F.A., & Donnelly, M. (eds). Research and Management Techniques for the Conservation of Sea Turtles. IUCN/SSC Marine Turtle Specialist Group Publication No. 4, 1999. pp 12-14.
Carr, A., Meylan, A., Mortimer, K., Bjorndal, K., & Carr, T. (1982). Surveys of sea turtle populations and habitats in the Western Atlantic. NOAA Technical Memorandum NMFS-SEFC-91. 82 pp+maps.
Eckert, K.L. & Hodge, K.V.D. (In review). WIDECAST Sea Turtle Recovery Action Plan for Anguilla. WIDECAST and UNEP-CEP Technical Report Series. Kingston Jamaica. 66 pp.
Epperly, S. (2000). Introduction to the Workshop. In: Bjorndal, K.A. & Bolten, A.B. (eds). Proceedings of a Workshop on Assessing Abundance and Trends for In-water Sea Turtle Populations. US Dep. Commer. NOAA Tech. Mem. NMFS-SEFSC-445, pp 1-2.
Godley, B.J., Broderick, A.C., Campbell, L.M., Ranger, S., & Richardson, P.B. (2004). An assessment of the status and exploitation of marine turtles in Anguilla. In: An assessment of the status and exploitation of marine turtles in the UK overseas territories in the Wider Caribbean. Final Project Report for the Department of Environment, Food and Rural Affairs and the Foreign and Commonwealth Office. pp 39-77.
Lagueux, C.J. (2001). Status and Distribution of the Green Turtle, Chelonia mydas, in the Wider Caribbean Region. In: Eckert, K.L. & Abreu-Grobois, F.A. (eds). Proceedings of the Regional Meeting: "Marine Turtle Conservation in the Wider Caribbean Region: A Dialogue for Effective Regional Management", Santo Domingo, 16-18 November 1999. WIDECAST, IUCN-MTSG, WWF, and UNEP-CEP. pp 32-35.
Meylan, A.B. (1983). Marine Turtles of the Leeward Islands, Lesser Antilles. Smithsonian Inst. Atoll Res. Bull. 278: 1-43.
Meylan, P. & Meylan, A. (1999). Procedures Manual for the Bermuda Turtle Project. Bermuda Aquarium Museum and Zoo, Caribbean Conservation Corporation, Florida Fish and Wildlife Conservation Commission, and Eckerd College. 36 pp.
Musick, J.A. & Limpus, C.J. (1997). Habitat Utilization and Migration in Juvenile Sea Turtles. In: Lutz, P.L. & Musick, J.A. (eds). The Biology of Sea Turtles. CRC Press, Boca Raton. pp 137-163.
Testudo Volume Six Number Two 2005
Top