Stuart McArthur, BVetMed, MRCVS
Holly House Veterinary Surgery, 468 Street Lane, Moortown, Leeds, LS17 6HA
Based on a presentation to the Northern Symposium at Chester Zoo on 15th October 2005
Anaesthesia and surgery go hand in hand. We can perform many complex surgical procedures on tortoises because we have already learnt so much about how to anaesthetize them safely.
In some public internet forums and discussion groups, I have come across tortoise keepers who suggest that you can perform some surgical procedures upon tortoises without an anaesthetic. Unfortunately this is because they have become familiar with the fact that reptiles like chelonians do not show pain very clearly. I have heard suggestions that you can just pin chelonians down and then conduct surgical procedures such as ear abscess curettage upon them without even using a vet, let alone any anaesthetics. In fact this is a big mistake. In reality, because chelonians do not show pain, we should actually spend more time finding out how to protect them from it.
Because we can now anaesthetise chelonians efficiently and reliably, and have a selection of pain relief drugs easily available, a whole new sphere of surgical procedures has opened up to us.
When do we anaesthetise these animals?
We do it for surgery, for injuries, for managing their diseases and for diagnosis. For example, if you are presented with a sulcata tortoise of 30kg and you want to examine its mouth, that will not be possible; if you have a snapping turtle you can do practically nothing at all; but if you can anaesthetize the animal you will have a better chance of deciding what is wrong with it. When examining difficult patients, digital photographs can be taken while you do the examination and you can review them later.
Historically, cold temperatures were used as a means of immobilisation for operating, but unfortunately simply immobilising the animal does nothing about its pain. Almost every single thing the temperature loss does to the animal compromises it and gives it less chance of recovery. If it has a wound, then chilling will handicap the animal’s ability to repair it, so is not helpful at all; in this country it is illegal, but in international Internet discussions it may still be quite commonly mentioned.
Which animals are suitable candidates for anaesthesia?
Often the patient will be unstable because of a life-threatening incident such as a lawn mower injury, so it may seem that we have to anaesthetise them quite quickly. If the condition is not life-threatening, ideally we like to stabilise the patient first; this is another reason why we can do much more with tortoises and turtles nowadays, because we have learnt to stabilise them before we anaesthetise them. We have protocols for many common conditions, and we are familiar with so many cases that we can predict most of the surgical scenarios.
An animal that has evolved from something that can dive, such as a marine turtle, has the ability to hold its breath for a long time and respire anaerobically. Similarly, an animal that has the ability to hibernate has evolved to survive under extreme conditions. These factors mean that chelonians are good surgical candidates as they are unlikely to die during anaesthesia.
How do we anaesthetise a tortoise or turtle?
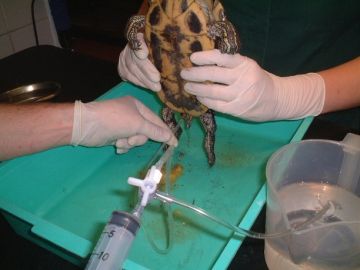
Option 1: Total intravenous injectable anaesthesia. The animal will be anaesthetised following an intravenous injection. If it starts to wake up you can just inject some more of the drug. You can ventilate the animal with the surrounding air.
Option 2: Injection plus volatile agent. The basic option above is quite safe, but with this option you can also ventilate the animal with a volatile agent and give it oxygen to improve its chances of survival. With automatic ventilation, which is standard with reptiles, you can have consistency, predictable recovery and are providing ventilatory support during the recovery phase. Otherwise, a person might need to be with the animal for several hours, which is very time consuming. With an automatic ventilator the vet or nurse could leave the animal ventilating and do other things as they monitor it.
Option 3: Balanced anaesthesia. There are great advances taking place in pharmacology now where you give a combination of chemical agents. This gives you a combination of the benefits of all the components, but the side effects of them are divided. A lot of information is coming out of the American universities at the moment, where they give mixtures of anaesthetic drugs to animals and publish success rates in their literature. This is legal in the USA, and we can use their results for our anaesthetic procedures on animals in general practice, whereas in Britain we are not allowed to try drugs unless they have a history of being relatively safe.
What drugs do we use?
Saffan: this can only be obtained in Britain, and is probably one of the safest anaesthetic agents for tortoises. It is wonderful because it is very stable, predictable and simple. You can give 10mg/kg intravenously to anaesthetize the animal for 20 minutes, and if the patient wakes up you can give it some more; you can continue incrementally dosing the animal, knowing that if the effect has not worn off in 20 minutes something has gone wrong, and you must work out why the patient has become unstable. In procedures that are going according to plan, it is very unusual to lose an unstable or even a critical patient with that type of protocol. It is also very cheap.
Propofol: this is used internationally in most small animal practices. The problem is that when you top it up (as with Saffan) because the animal is waking up, the second recovery is 40 minutes, and the third may be 3½ hours. Many veterinarians disagree with this and they find that recoveries are not prolonged. Sadly there have been no quantifiable experiments to confirm either opinion. If relying on this as the main agent, the more doses you give the more the animal’s condition may be compromised. However, it is quite reliable if you give it once and continue with an inhalation agent.
Ketamine: this had been used for 30-40 years and then went out of favour, but now we have gone full circle and it is often used in combination with other drugs. Given at a very low dose intravenously, it is very reliable for giant species. In cases where you struggle to handle the animal for intravenous routes, such as sulcatas and other giant species, where you can only just get hold of a piece of skin, you can give a drug combination intramuscularly; this works brilliantly as a knock-down. We now have protocols including reversal agents (for some cocktails) so you can revive the animal, making the procedure safe and controllable.
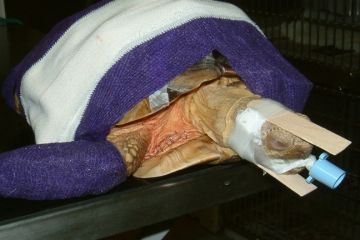
Once the animal is asleep it cannot ventilate, so we have to create an airway by intubating through the mouth, which is probably clamped shut. We then go on to automatic ventilation and use volatile anaesthetics, which are safer than giving more injectable drugs:
Halothane is safe and reliable, but has a comparatively poor human safety profile for veterinary staff.
Isoflurane, the most commonly used agent, is very safe and reliable.
Sevoflurane is similar to isoflurane and is now popular in America.
Monitoring and maintaining the anaesthetised animal
A big investment in expensive equipment, such as pulse oximetry and Doppler which would be £2,000 or £3,000, improves safety. A ventilator alone would be £600.
The chances of recovering the cost are not good unless you use the equipment for other species besides exotics. Without this investment it is still safe to anaesthetise animals; simple ventilation is easy because moving the legs in and out compresses or expands the respiratory compartment, forcing ventilation; this is the alternative to a machine. However, automatic ventilation is better, and larger machines are available for marine turtles and large animals.
Temperature maintenance
Temperature is very important. If you chill the patient it may suffer prolonged anaesthesia. The protocols to try to keep the animal stable at 26° C are: heat the room, start with a warm operating table, keep the patient warm, give fluids at the right temperature, and use a core temperature probe to watch if the temperature is dropping . Minimising operation time is also vital. Since anaesthaesia automatically causes chilling, bottles of hot water can be placed around the animal. This is equally important during recovery, which can be prolonged in a chilled animal.
Diving species and blood shunting
There is a slight problem with animals that can dive or have evolved from diving animals: if a turtle goes into water it does not need to breathe or use its lungs, so it cuts the lungs out of its circulation. Under anaesthetic the turtle cut out the lungs and the effect of a volatile anaesthetic is unreliable. When you want them to wake up, rather than oxygenating them, you need to deprive them of oxygen because of this dive reflex and vascular shunt system; this encourages them to breathe and remove any anaesthetic gases. Anaesthetic machines normally have oxygen on them, so you are better off manually ventilating the animal in air rather than leaving them on the machine, which seems bizarre, but turtles benefit from this. Providing carbon dioxide also stimulates them to take deeper breaths.
Pain relief
Pain relief during recovery is based on anecdotal data for common painkillers. It is very hard to assess because it is difficult to interpret signs of pain – how active should the animal be? Is its behaviour normal? Is it reclusive? Is it failing to eat? Unless the observer is familiar with that animal, you can give drugs without really knowing whether or not this is benefiting the animal. Current trends show that the common painkiller meloxicam is the right choice given what is available to us. This is the only pain killer to have been studied in reptiles at this point in time.
Euthanasiais included in this section because it uses anaesthetic agents and it is, in a sense, a way of relieving pain. Chelonians are hard to kill, because if you use the same agents as for a cat or dog they might go into an inert state for many hours, metabolise the agent, and then wake up because of their physiological make-up and ability to respire anaerobically. So after inducing a state of unconsciousness whereby the animal is unaware of what is going on, a crucial act such as pithing has to be carried out to make recovery impossible. This is the physical destruction of the brain.
SURGERY
Once you are confident that anaesthetics are safe (and that confidence may be hard to get) there are several considerations in actually proceeding. Firstly, why bother to operate on these crazy primitive animals? Well, if people love their pets, and care for them, they are prepared to pay for the service, and vets acquire a lot of experience from this. In Britain, anaesthaesia is used widely for disease and trauma management, plus occasionally for diagnosis.
I shall now give some examples of interesting procedures.
Coeliotomy
Because of the surrounding shell, there are different choices for approaches to the coelomic cavity. These are: centrally through plastron (plastron osteotomy), by making a trap door; or entering to the side through soft tissue round the outside of the plastron, for animals with a proportionately smaller plastron such as marine turtles (this technique is not transferable to terrestrial species). For a loggerhead turtle, for example, we could get an arm right inside the animal through the soft tissue in front of its hind legs. It is better for aquatic animals to operate through soft tissue not shell, because skin heals much faster than shell, and the animal must go back into water as soon as possible for normal unchallenged recovery to take place.
Simple equipment can be quite adequate, such as a dremmel with extension and diamond cutting blade for £80 – 90 from Homebase. In a university environment there is access to equipment such as oscillating saws, but I still use a normal basic system.
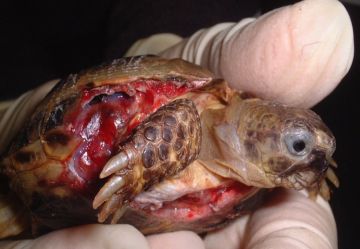
To undertake surgery, for example in a sulcata tortoise with a fractured shoulder, we need to decide how to access the surgical site. Once the patient is anaesthetized, its position is unimportant to it because it is not distressed, so rather than keeping it horizontal you can strap it into a more convenient but unconventional position.
If the animal is upside down, all the weight is on its chest and lungs under the dorsal surface, so the animal is tipped sideways to open up the respiratory compartment. We can ventilate it for operating. If we lean on the animal while we are concentrating on other things we might break its legs, as they are flaccid and quite heavy, so we tape them in place to avoid injury. The animal may be very dirty, so we must clean it or wrap up the dirty part. We may lose our landmarks while operating, so we can mark points out on the animal with a pen before we begin.
When entering the animal through its plastron, we have to consider the problems of heart, bladder, pelvis and pectoral girdle, so we have a very narrow window of access to the body. Once we have decided on the approach we score the access route, and drill into it cutting the flap at an angle so it does not fall into the animal.
Closing the coeliotomy:
Healing comes mainly from the coelomic membranes if you preserve them when you enter the animal. I tend to replace the trap door made during entry, but if you are feeling lucky, you can even throw the flap away and just utilise the coelomic membrane as a natural bandage while bony shell re-grows. I find the bone offers good protection to this membrane. Fibreglass is then used to cover the wound, or we can use sutures, dental acrylics or screws and wires.
Semi-aquatics such as red-eared terrapins with a proteinaceous plastron do not take kindly to fibreglass or flap replacement. They often build up proliferative granulation tissue if you put the flap back. In these cases, wounds are better left open with a dressing over the membrane. A new plastron can grow in 6 – 8 weeks with a patch of x-ray film punched on as a protective covering.
Coeliotomy complications:
In a small percentage of cases, the pericardium or bladder can be damaged, so a few simple cases are lost by accidental puncture. Some will survive if repair has been made to the surgical damage, but these tissues are so delicate that occasional damage upon coelomic entry can be unavoidable.
Egg retention
The solution to this is often environmental; the body clock is mis-timing, and surgery is not normally needed, but we must work out what changes can be made to the animal’s routine. Ovulation can be induced with drugs, but sometimes we need to operate to remove the eggs. They can be in the bladder, or may be free in the coelomic cavity if the uterine horn has ruptured, or even stuck to the oviduct if the egg has passed through too slowly.
An x-ray shows the status of the process; old eggs will usually be over-calcified, and a build-up of eggs probably means some are stuck to the wall of the oviduct. They could possibly be manipulated out, but the situation must be weighed up as to whether an operation is worthwhile both for the owner and for the tortoise. If the eggs are in the bladder they may have urates stuck to them, in which case the animal may die unless they are removed.
An egg in the bladder:
The bladder is a dirty organ and cannot be easily exteriorized; stay sutures are used to bring it to the edge of the incision, and then the eggs are removed to the outside of the animal. The eggs can be raised with a teaspoon or tongs. They must not touch the inside of the coelomic cavity, because an egg may be foul with pus instead of normal yolk. This type of operation is a gamble for the owner, but has often been successful.
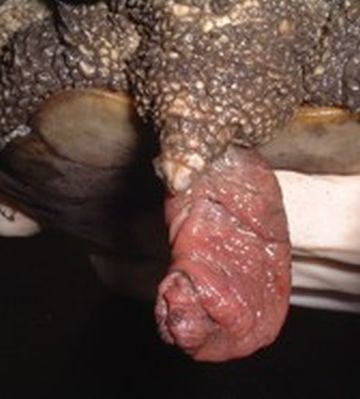
Oversized eggs:
A giant egg can be stuck in the pelvic canal. In this case, we anaesthetise the animal, inspect the cloaca, then aspirate and remove the eggs. Lavaging out may be sufficient, and toxic collapsed animals can be surprisingly hardy and survive this. Again, this situation has probably been caused by wrong husbandry, with the absence of a haul-out area in the case of aquatic species.
In conclusion, there is also quite a big input into the development of anaesthesia and surgery from conservation projects, because there is a lot of work which involves examining wildlife. Foreign universities, mainly in the USA, keep the momentum of research into reptiles going. We learn a great deal from this, which can be brought not just into conservation work to try and save species, but also into pet management to try to save animals that are suffering.
Photos by Stuart McArthur.
Testudo Volume Six Number Three 2006
Top