Roger Meek
7 Rue Georges Clemenceau, Chasnais, France.
E-mail: Rogermeek85{at}aol.com
Introduction
Information on the composition of natural diets in animals can provide insight into a species’ role in an ecosystem and contribute to effective captive husbandry and breeding programmes. Under natural conditions, a critical aspect of nutritional behaviour is the level of energy expended to access food resources, which may vary depending on whether a species is a dietary generalist or specialist. For example, in herbivorous reptiles a generalist feeder will usually select a range of plants to satisfy overall dietary requirements, whilst a specialist may have to forage widely for a more limited food plant range (review in Stephens & Krebs, 1986). Additionally, herbivores must also be aware of plants that contain toxic substances, which if consumed, at the very least, may impose costs for digestion (Freeland & Janzen, 1974). Such plants are usually avoided but this is not always the case, particularly if they contain essential nutrients (Zahorik & Haupt, 1977). Foraging theory has sought to explain dietary behaviour in animals, but was concerned mainly with insectivorous lizards and birds (MacArthur & Pianka, 1966) – it is less easily applied to herbivorous tortoises, where diets are more in terms of selection of a mixture of nutrients. However, one of the advantages of the theory is that it allows predictions to be tested in a somewhat subjective area of ecology and enables insight into interspecific differences in dietary behaviour. The nutritional behaviour of Hermann’s tortoise Testudo hermanni has received limited attention in field studies, particularly in respect to defining the degree of dietary specialisation or generalisation. The purpose of this paper is to provide baseline information on dietary selection and test for dietary strategy.
Methods and materials
The results are derived from a re-analysis of data collected during research on the ecology of T. hermanni, in what was at the time Yugoslavia. Field work was carried out during spring (May), summer (late May – June) and autumn (September – October) on several populations, the first during 1978 near the town of Budva in Montenegro (Meek & Inskeep, 1981) and others in Montenegro and Croatia during 1983, 1984 and 1986. The core of the research concerned population ecology (Meek, 1985; 1989) and thermoregulatory behaviour (Meek, 1984; 1988). Field methods were given in detail in the original papers (Meek, 1985; 1989). Briefly, this employed the spot measurement method, where daily routine patrols were made of the study sites and a series of measurements taken from each tortoise located, after which it was marked and released. Information included sex, body mass, carapace length (straight line), behaviour and body temperatures (cloacal, measured with a digital thermometer). Habitat plants were collected routinely and samples taken of any plant observed being consumed by a tortoise. Food plant data are in the form of numerical frequencies rather than volume.
Statistical methods. The Simpson’s Diversity Index was employed to define feeding strategy. The values in the index range from 1 to 0, with 1 indicating a broad based generalist diet – essentially they consume virtually any plant they come across – and 0 a very restricted specialist diet. The formula has the form:
D = 1- Σ[n (n-1)] / [N(N-1)]
where food plant diversity D is determined from the number of plants consumed from any plant family n within the total number of plant families being consumed N. Tests for differences in feeding strategy between populations have been made using t-tests applied to the index variances (Brower & Zar, 1984).
Results
Figure 1 shows the results with the numerical frequencies of each group of plants expressed as percentages of the total food plant sample in each region. One specimen could not be identified and so the total plant sample from Meek (1985; 1989) and the preliminary data from Meek and Inskeep (1981) was 62. Wide ranges of plants were consumed in both areas with D-values from two populations in Croatia of 0.75 and 0.82 and a single population in Montenegro of 0.93. The Montenegrin data had greater diversity (although also a larger sample size) and t-tests showed that the differences between the two areas were significant; t-values of Montenegrin versus Croatian samples from 3.7 to 9.0 and P-values from 0.0001 to 0.002. However, there was an apparent preference for legumes (mostly Medicago sp.) in both regions which was slightly greater in Montenegro (Montenegro, 60%; Croatia, 50%) but a z-proportional test indicated that the difference was not significant, z = 0.76, P = 0.44. In Montenegro, plants containing alkaloids or other toxins were present in 16.7% of the total sample, which increased to 26.2% if species containing other potentially damaging compounds are included. The Chenopodiaceae, for example, are known for high nitrate concentrations and Aristolochiaceae for the presence of saponins (Cooper & Johnsone, 1984). No toxin-containing food plants were found in the Croatian samples. Table 1 gives a list of food plants containing toxins.
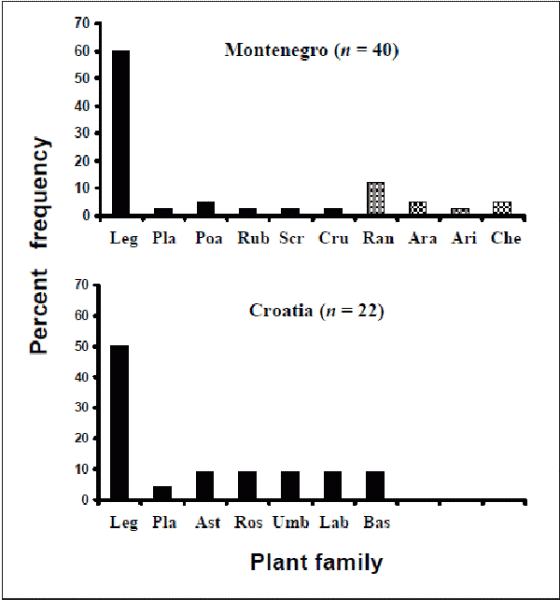
Fig. 1. Food plant selection in T. hermanni in Montenegro and Croatia expressed as % frequencies of the total samples from each region. Chequered histograms represent plant types with toxic or potentially damaging compounds. Keys for plant families are: Leg, Leguminosae; Pla, Plantaginaceae; Poa, Poaceae (= Gramineae) ; Rub, Rubiaceae; Scr, Scrophulariaceae; Cru, Cruciferae; Ran, Ranunculaceae; Ara, Araliaceae; Ari, Aristolochiaceae; Che, Chenopodiaceae; Ast, Asteraceae (= Compositae); Ros, Rosaceae; Umb, Umbelliferae; Lab, Labiatae; Bas, Basidiomycetes. See text for further details
Table 1. Food plant species of Testudo hermanni in Montenegro containing toxins or potentially damaging compounds. Data re-drawn from Meek (1985, 1989).
| Family | Genus
| Species
| Compounds
|
|---|
| Ranunculaceae | Clematis | vitella | alkaloids |
| Ranunculaceae | Clematis | - | alkaloids |
| Ranunculaceae | Ranunculus | acris | alkaloids |
| Araceae | Arum | italicum, maculatum or orientalis | alkaloids |
| Araceae | Arum | italicum, maculatum or orientalis | alkaloids |
| Araliaceae | Hedera | helix | saponins |
| Chenopodiaceae | Chenopodium | - | high nitrate concentrations in leaves |
| Aristolochiaceae | Aristolochia | - | acids may cause renal failure and cancers in humans |
Discussion
Although it is recognised that information about food plants without detailed knowledge of potential food plant availability have less value, such information is nevertheless a useful preliminary stage in understanding feeding ecology (Rall & Fairall, 1993; Loehr, 2002). The methods employed were also unable to determine the quantities of each plant ingested at any one observation. However, the direct method of sampling herbivorous animal diets, although time consuming, particularly when studying low energy budget ectotherms where feeding may be infrequent, is more accurate compared to faecal sampling (e.g. El Mouden, et al., 2006), where there is a high potential for error due to different digestibility of different plant material and hence difficulties in identification. The present results indicate a strategy of nutritional generalist but with selection for certain plant groups when they are available (e.g. Denise Dearing & Schall, 1992: Haxhiu, 1995). The Fabaceae (= Leguminosae, i.e. beans, clover, lupins etc) represent a dietary staple, probably due to their high nutritional content and easy assimilation (Jailwal & Singh, 2003). The grasses (Poaceae = Graminae), although abundant on all study areas, were rarely consumed; this indicates that although tortoises could quickly fill their stomachs they do not do so. Presumably, the nutritional benefits of seeking out less abundant species with easier energy assimilation (e.g. legumes) outweigh the foraging and locomotory costs to locate them. Food plant diversity may be influenced by factors other than availability. In autumn in Croatia, food plant diversity was reduced to 70% of that found in summer, and associated with simultaneous reductions in locomotory movement (15.3% in autumn versus 30.4% in summer) and feeding frequency (9% in autumn compared to 16.8% in summer). Reduced locomotory activity and feeding frequency were in turn associated with lower body temperatures (Meek, 1988). Compared to certain other tortoises, particularly those from arid zones (e.g. Nagy & Medica, 1986; Lagarde et al., 2003), feeding frequencies in T. hermanni were relatively high, but the spot measurement method may produce different results to studies where tortoises were followed individually and hence not strictly comparable. However, other factors could explain the differences, for instance thermal constraints in different habitats or size differences between species.
A broad based diet has been found in T. hermanni populations where there are reasonable sample sizes. For example, Haxhiu (1995) found that Albanian T. hermanni consumed mainly leguminous plants from the sub-family Papilionaceae, although cultivated species (e.g. Cucurbitaceae – cucumbers etc), fallen fruit and some animal matter formed part of the diet. A crude calculation of the Albanian data gives a minimum diversity index of 0.67, which is in good agreement with the present results. Feeding on what is nutritious and readily available is common in tortoises; for instance, mushrooms that normally grow in damper conditions were consumed in autumn by T. hermanni in Croatia (Basidiomycetes, see Fig. 1). Several species of tortoises are known to feed on mushrooms, which may have a higher energy value than leaves (e.g. Rodriguez Bayona & Rylander, 1984; Hailey et al., 1998).
Species related to T. hermanni may have a more restricted diet. For instance, plant food diversity was lower in the steppe tortoise Testudo (= Agrionemys) horsfieldii (Lagarde et al., 2003). Apparently this species adjusts its diet in response to plant availability, feeding almost exclusively on the most abundant plants, although similarly to T. hermanni the Poaceae were generally avoided. Lagarde et al. (2003) attributed this to nutrient problems, commenting that dry grasses cause negative water and nitrogen balances. This may not be important in T. hermanni living in moister habitats in the Balkans. Grasses are a dietary staple in the leopard tortoise Stigmochelys
(= Geochelone) pardalis (Milton, 1992). Recent data from Morocco indicated a narrow diet in Testudo graeca where the Asteraceae (= Compositae) and Fabaceae formed 70% of the faecal pellets (El Mouden et al., 2006). However, the Moroccan data were from an unusual environment and may not be typical – food plant diversity in T. graeca was broader in other areas (Andrea, 1987).
A notable feature of many reptilian herbivores is the consumption of plants containing chemicals that are toxic for mammals (e.g. Nachman & Olsen, 1983; Olsen et al., 1983) including species from the Ranunculaceae (Lagarde et al., 2003). What benefits are conferred by this behaviour? Lagarde et al. (2003) proposed that it enables a reduction in competition with herbivorous mammals, although earlier studies have suggested that toxins incur a high metabolic cost in digestion (Freeland & Janzen, 1974), which may cancel out any benefits. Longepierre & Grenot (1999) reported a diet shift in T. hermanni from one based on the Ranunculaceae, containing high levels of the toxin ranunculin, to toxin-free Asteraceae after treatment with vermifuge. Presumably, the assumption is that naturally available vermifuge was involved in removing or reducing parasite loads and if there are high metabolic costs, they are outweighed by the benefits. This is an unexpected result and ought to be further investigated, since internal microbe and nematode populations are normally important for digestion in herbivores and damage to the intestinal fauna could adversely affect their health (e.g. Cooper, 1980; Innes, 2001; Wadding et al., 2004). Indeed, Iverson (1982) has suggested that certain reptilian herbivores may have evolved specialised partitioned colons to facilitate high densities of nematodes, bacteria and protozoa, and Tracy et al. (2006) that optimally herbivores should avoid changing food plants to avoid potential damage to the internal microbe community.
Acknowledgments
Mr T. Crosby at the University of Leeds, Department of Plant Sciences (now Faculty of Biological Sciences) identified plant samples and drew attention to species with alkaloids described here and in the 1985 paper. Comments by an anonymous reviewer improved the manuscript. Christine Tilley drew attention to recent changes in plant classification. The research was funded by the British Ecological Society.
References
Andrea, A.C. (1987). Ecologia dinamica poblacional de la tortuga mora, Testudo graeca, en Donana. Thesis doctoral, University of Seville.
Haxhiu, I. (1995). Results of studies on the chelonians of Albania. Chelonian Conservation and Biology 1: 324-326.
Brower, J.E. & Zar, J.H. (1984). Field and Laboratory Methods for General Ecology. Brown, Dubuque, IA.
Cooper, J.E. (1980). Antibiotics and chelonians in captivity. British Herpetological Society Bulletin 2: 44.
Cooper, M. R. & Johnson, A. W. (1984). Poisonous plants in Britain and their effects on animals and man. Her Majesty’s Stationery Office, London, England. 305 pp.
Denise Dearing, M. & Schall, J.J. (1992). Testing models of optimum diet assembly by a generalist herbivorous lizard Cnemidophorus murinas. Ecology 73: 845-858.
El Mouden, E.H., Slimani, K., en Kaddour, K., Lagarde, F., Ouhammou, A. & Bonnet, X. (2006). Testudo graeca graeca feeding ecology in an arid and overgrazed zone in Morocco. Journal of Arid Environments 64: 422-435.
Freeland, W.J. & Janzen, D.H. (1974). Strategies in herbivory in mammals: the role of plant secondary compounds. American Naturalist 108: 269-289.
Hailey, A., Chidavaenzi, R.L. & Loveridge, J.P. (1998). Diet mixing in the omnivorous tortoise Kinixys spekii. Functional Ecology 12: 373-385.
Innes, C. (2001). Medical issues affecting the rehabilitation of Asian chelonians. Turtle and Tortoise Newsletter 4: 14-16.
Iverson, J.B. (1982). Adaptations to herbivory in Iguanine lizards. In: Iguanas of the World; Their Behaviour Ecology and Conservation (eds. Burghardt, G.M. & Rand, A.S.). Noyes, New York, pp. 60-76.
Jaiwal, P.K. & Singh, C.R. (2003). Applied Genetics of Leguminosae Biotechnology. Kluwer Academic Publishers, Dordrecht, Netherlands.
Lagarde, F., Bonnet, X., Corbin, J., Henen, B., Nagy, K., Mardonov, B. & Naulleau, G. (2003). Foraging behaviour and diet in an ectothermic herbivore: Testudo horsfieldii. Ecography 26: 236-242.
Loehr, V.J.T. (2002). Diet of the Namaqualand speckled padloper, Homopus signatus signatus, in early spring. African Journal of Herpetology 51: 47-55.
Longepierre, S. & Grenot, C. (1999). Some effects of intestinal nematodes on the plant foraging behaviour of Testudo hermanni in the south of France. In: Current Studies in Herpetology (eds. Miaud, C. & Guyetant, R.). Societas Europaea Herpetologica, pp. 277-284.
MacArthur, R.H. & Pianka, E.R. (1966). An optimal use of a patchy environment. American Naturalist 100: 603-609.
Meek, R. (1985). Aspects of the ecology of Testudo hermanni in southern Yugoslavia. British Journal of Herpetology 6: 437-445.
Meek, R. (1984). Thermoregulatory behaviour in a population of Hermann’s tortoise (Testudo hermanni) in southern Yugoslavia. British Journal of Herpetology 6: 387-391.
Meek, R. (1988). The thermal ecology of Hermann’s tortoise (Testudo hermanni) in summer and autumn in Yugoslavia. Journal of Zoology, London 215: 99-111.
Meek, R. (1989). The comparative population ecology of Hermann’s Tortoise, Testudo hermanni, in Croatia and Montenegro, Yugoslavia. Herpetological Journal 1: 404-414.
Meek, R. & Inskeep, R. (1981). Aspects of the field biology of a population of Hermann’s tortoise (Testudo hermanni) in southern Yugoslavia. British Journal of Herpetology 6: 159-164.
Milton, S.J. (1992). Plants eaten and dispersed by adult leopard tortoises Geochelone pardalis (Reptilia: Chelonii) in the southern Karoo. South African Journal of Zoology 27: 45-49.
Nachman, R.J. & Olsen, J.D. (1983). Ranunculin: a toxic constituent of the poisonous range plant buttercup (Ceratocephalus testiculatus). Journal of Agriculture and Food Chemistry 31: 1358-1360.
Nagy, K.A. & Medica, P.A. (1986). Physiological ecology of desert tortoises in southern Nevada. Herpetologica 42: 73-92.
Olsen, J.D., Anderson, T.E., Murphy, J.C. & Madsen, G. (1983). Bur buttercup poisoning of sheep. Journal of the American Veterinary Medicine Association 183: 538-543.
Rall, M. & Fairall, N. (1993). Diets and food preferences of two South African tortoises Geochelone pardalis and Psammobates oculifer. South African Journal of Wildlife Research 23: 63-70.
Stephens,W.S. & Krebs, J.R. (1986). Foraging Theory. Princeton University Press.
Rodriguez Bayona, L.O. & Rylander, M.K. (1984). Notes on the biology of the tortoise Geochelone denticulate L. in Peru. Amphibia-Reptilia 5: 323-327.
Tracy, C.R., Nussear, K.E., Esque,T.C., Dean-Bradley, K., DeFalco, L.A., Castle, K.T., Zimmerman, R.E., Espinoza, R.E. & Barber, A.M. (2006). The importance of physiological ecology in conservation. Integrative and Comparative Biology: 1-15. (open access).
Wadding, T., Mann, S.L., Davies, M. & Meek, R. (2004). Possible effects of antibiotic therapy on digestion in a Solomon Island Skink, Corucia zebrata. Herpetological Bulletin 89: 2-3.
Zahorik, D.M. & Haupt, K.A. (1977). The concept of nutritional wisdom: applicability of laboratory learning models to large herbivores. TX: Baylor University Press, pp. 45-70.
Editor’s note: Taxonomic changes have occurred with some plant families, and the Latin names now all end with -eae, losing some familiar ones such as the Graminae. However, in many cases the earlier names are still in use, especially where colloquial names have entered the language, such as the legumes and the labiates.
Testudo Volume Seven Number Two 2010
Top

