Sofie Rogers BSc (Hons) Zoology, 5 Rosehall Place, Haddington EH41 4ED
Based on a presentation to the BCG Northern Symposium at Chester Zoo on 17th October 2009.
Introduction to Loggerheads, Greens and Turtlewatch.
In the Mediterranean, two species of sea turtle nest along the coasts of Greece, Libya, Israel, Syria, Turkey and Cyprus. These are green (Chelonia mydas) and loggerhead turtles (Caretta caretta), both classified as Endangered by the IUCN (International Union for Conservation of Nature). Data on the age, size, growth rate and reproductive output of individuals within these species remains sporadic and so, therefore, does our understanding of the true population status of these long-lived reptiles. As with any species with a long and complex life history, the value of long-term data sets is unquestionable.
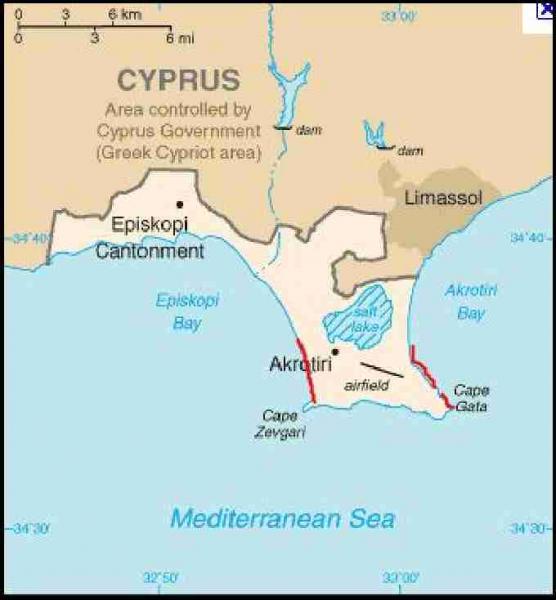
Cyprus is located in the eastern Mediterranean close to the coast of Turkey. The sea turtle conservation programme Turtlewatch monitors beaches surrounding RAF Akrotiri (Fig. 1) and has been running since 1991, with Glasgow University volunteers involved since 1998. The Turtlewatch expedition is run in conjunction with RAF Akrotiri and Glasgow University Exploration Society. Downie et al. (2003) published nesting results at Akrotiri from 1998 to 2002 and concluded that although turtles do not nest in large numbers at Akrotiri, it is still an important conservation area given the general declining population trends of these species. They also stated that the collection of long term data on these species is very important. The ability of undergraduate students to collect, record and analyse data forms a key part of Turtlewatch at Akrotiri.
Reproductive ecology of loggerheads and greens
In order to lay, female adult turtles return to the areas in which they were born (natal areas) at approximately 20 years of age (Ackerman, 1997) and most marine turtles are non-annual breeders. Broderick et al. (2003) found that both species have a remigration interval (number of years between breeding seasons for an individual female) of 2-3 years and that the reproductive efforts of a female depend on how big she is and also the nutrients available in any given year.
Threats
Turtles in the marine environment experience detrimental anthropogenic effects during both the adult and juvenile life history stages. The degradation of their foraging and nesting grounds, accidental by-catch in long-line fisheries (Sales et al., 2008) and pollution have all had large scale effects on adult populations of all species. Sound, litter and light pollution have the effect of either disturbing adults trying to nest, or confusing hatchlings attempting to get to the sea. Emerging hatchlings use light as their main orientation cue, so entire nests of hatchlings can be lost if there is a hotel or street lighting behind the beach.
Another emerging threat to turtles is global warming. Rising temperatures not only mean changes to their food supply and habitat, but will also affect hatchling development and produce a female-biased population if no compensatory behaviour takes place.
The effects of temperature on turtles
As turtles use temperature dependant sex determination (TSD), the temperature of the nest affects the ratio of males to females. Their sex is determined during the middle third of the incubation period. Research conducted in the Mediterranean discovered that the pivotal temperature to produce a 1:1 ratio for both species is just below 29°C (Godley et al., 2001a), with some small regional deviations. The bias towards all female hatchlings increases in nests warmer than this threshold value and conversely the bias to all males increases as the temperature decreases. If temperatures continue to rise with global warming, then a high female biased sex ratio will emerge and female turtles will find it increasingly difficult to locate males with which to mate (Godley et al., 2001b).
Incubation temperature has also been shown to influence size and locomotor performance (Booth et al., 2004; Mickelson & Downie, 2010; Glen et al., 2003; Glen et al., 2005) of juvenile turtles, while Booth & Astill (2001) demonstrated that incubation temperature influences the stroke rate of flippers during swimming in green hatchlings. Consequently, any characteristics that would assist in predator avoidance or increasing swimming speed are most likely to be naturally selected for, as hatchling morphology must allow for both a successful race to the sea and swimming. Data on hatchling morphology and terrestrial speed were collected in 2008 and 2009 in order to study the effects of temperature on these in green and loggerhead hatchlings, with the results being presented elsewhere.
Sites
There are six main beaches at Akrotiri (located within the Sovereign Base Area (SBA) – land occupied by the British military forces in Cyprus) which were patrolled during the 2008-2010 seasons on a nightly basis by members of the Glasgow University Turtlewatch expedition. Cove 1 and Cove 4 are the two main nesting beaches.
Cove 1 (34°35’01”N: 32°56’28”E) is the more southerly and is approximately 360m in length and 55m in width. It is separated from Cove 4 by cliffs and a man-made harbour, which increased in size in early 2008 to accommodate more local fishing vessels from Akrotiri village. Cove 4 (34°35’20”N: 32°56’30”E) is approximately 540m long and 130m wide. Coves 2,3,5 and 6 often have multiple false crawl attempts (where the females have emerged but not laid) but few nests. This may be because shallow sand means that nesting females encounter rock quickly.
Turtlewatch also monitors the beaches on the other side of the Akrotiri peninsula, on the RAF base itself. These have fewer nesting attempts on them, but do produce a few viable nests annually. In addition to this, in 2009 a new nesting beach was discovered on the base on the south east of the peninsula and five loggerhead nests were laid.
Nest excavations are performed on all nests as they present a unique opportunity for Turtlewatch volunteers to increase the awareness of local Cypriots and military personnel about the difficulties faced by marine turtles, and their importance as part of the ocean ecosystem. We try to raise awareness about the detrimental effects that beach users can have on turtles (e.g. litter on the beaches, driving on the beach destroying nests, parties producing light that scares females and disorientates hatchlings, walking dogs that might dig up a nest etc). Volunteers can interact with members of the public and answer questions. We give presentations to primary school classes about turtles, the threats they face and what people can do to help them. Turtlewatch also runs a shop and information centre on RAF Akrotiri where merchandise is sold to raise funds for the project, staffed by project volunteers so members of the public can ask questions. Turtlewatch believes that education about turtles is a key step to promoting their conservation.
Methods
Monitoring of Nesting
As the nesting season takes place from early June until the end of September or early October, three groups of students were rotated during this time thereby allowing a greater number to participate. They were there for approximately five weeks each, while the two project leaders stayed for the duration of the season. The majority of turtle activity occurs at night so students were present on the beaches from 9pm to 6am. Patrols along the lengths of beaches 1-4 were carried out every two hours during the nesting season. The other beaches (5 & 6 and those on base) were checked every morning, as it was not possible to do so at night due to health and safety restrictions. During patrols volunteers searched for evidence of turtle activity, which consisted of tracks left by a turtle that had previously laid or one that was currently laying. False crawls (Fig. 2) were also recorded. During all nocturnal patrols, students used red light filters on their torches so as not to disturb the turtles.

Fig. 2. False crawl by loggerhead on Cove 4.
If a female was discovered laying (Fig. 3), she was closely monitored. Any distinguishing features, such as barnacles, were noted (as turtles were not tagged). Once a female had entered the ‘laying trance’, the curved carapace width (CCW) and curved carapace length (CCL) were measured using a measuring tape. If a female was not discovered laying, she was not measured, in order to avoid disturbance on her return to the sea. The position of the nest chamber was noted if a clutch was successfully laid. When a nest was found, a protective cage was placed on top to prevent predation by foxes (Vulpes vulpes). Deterrents were used around the nests, such as surrounding them with wooden sticks, wire, streamers and reflectors in an effort to reduce fox predation. A sign displaying a nest number and advising beach users not to disturb the nest, in both English and Greek, was placed next to the nest.

Fig. 3. Loggerhead with distinctive barnacle during oviposition on Cove 1
Monitoring of nest temperatures
We placed Gemini Tiny Talk temperature data loggers (TDL: Tinytalk, Orion Components Ltd, Chichester, UK. Precision 0.3°C) set up to record temperature at specific intervals, in several nests. TDL were inserted with minimal disturbance while egg laying was taking place and the female was in the ‘laying trance’. They were also inserted into any relocated nests (Fig. 4). Where possible, TDL were inserted into the top, middle and bottom of a clutch. However, for some nests it was only possible to insert one TDL. They were set up to take a temperature reading every four hours in 2008. In 2009 it was decided that they should record a temperature every hour to ascertain a more accurate temperature profile for a given clutch.

Fig. 4. Relocated nest with TDL inserted at the bottom of the nest
Monitoring of Hatching
When the hatching season began, usually in late July, beach patrols increased in frequency to every hour. Any nest due to hatch was checked at hourly intervals throughout the night to monitor for hatchling emergence. When fox disturbance of nests occurred, nests partially uncovered had any shell fragments removed and the remaining eggs were re-buried. When a nest hatched, volunteers escorted hatchlings to the sea to deter predators. If hatchlings appeared to be very disorientated by anthropogenic lights, they were guided by torches to the sea.
Further data collection
When hatchlings emerged from a TDL nest, 20 hatchlings were detained for study where possible. They were rinsed, air dried and then weighed with a spring balance and morphological measurements were taken using callipers. These measurements were taken in both 2008 and 2009. A runway was then created and each hatchling’s terrestrial speed was calculated from stopwatch timers. A detailed methodology and statistical analysis of these time trial results are presented elsewhere (Rogers, 2009). In 2010, data were collected on the ghost crabs (Ocypodes cursor) as they predate turtle hatchlings and are themselves endangered.
When possible we collected data to send to the Environmental Department of the Cyprus government on any dead turtles that washed up. Figure 5 shows a male loggerhead that was washed up onto one of the beaches in 2009. Results from the necropsy suggest that he was killed by a stab wound to his skull but it was not possible to determine how he obtained the injury.

Fig. 5. Male loggerhead found with stab wound to the head and abrasions on carapace, 2009.
Nest excavations
Excavations of the nest chambers were carried out by hand on all nests ten days after the first hatchling emergence in order to calculate the hatching success rate and to allow any TDL to be collected. The delay in the timing of excavations in 2009 and 2010 was a requirement of the Cyprus government. Excavations in previous years were carried out on all nests after three days. Figure 6 shows a public excavation taking place.

Fig. 6. Public excavation, Cove 1, 2010.
The data collection contributes to the long term data set held by Akrotiri Turtlewatch. The information collected from nests was: number of hatched eggs; number of infertile eggs (those showing no embryonic development); number of early stage development (Fig. 7); number of late stage development (Fig. 8); number of dead hatchlings in nest; and total number of eggs in nest. Any live hatchlings stranded in the nest, sometimes due to litter debris trapping them, were released (Fig. 9). In some cases, an entire nest would show no signs of embryonic development. This may be due to unfertilised eggs, or perhaps because of anthropogenic effects such as oil in the sand, which contaminates the eggs. Nest excavation data for the 2008-2010 period is presented for nests not depredated by foxes.

Fig. 7. Showing three stages of embryonic development categorised as ‘early development’ for recording purposes. Embryo on the far right shows egg yolk sac attached to the embryo.

Fig. 8. Showing ‘late development’ loggerhead embryos with yolk sacs. Note that left embryo appears to be lacking pigmentation.

Fig. 9. Green hatchling released from a nest makes its way to the sea.
Compilation of Data
Data from past expeditions and their reports are held by the University of Glasgow Library. Results were also held in a database by Turtlewatch. Every effort was made to standardise methodology, but it cannot be guaranteed for all of the expeditions. Nests that were depredated, waterlogged or relocated were excluded from analyses with the exception of annual results presentations.
Results
Loggerhead turtles nesting on Cyprus are the smallest breeding females reported for the species with CCL of 63-87cm (Broderick et al., 2003). At Akrotiri in 2009, the average loggerhead CCL was 71.6cm and the CCW was 63.4cm (n=13). Greens are typically slightly larger with CCL of 77-106cm (Broderick et al., 2003) and were found in 2009 at Akrotiri to have an average CCL 84cm and CCW 77cm (n=3).
Incubation times from 2008-2010 seasons vary from 45 to 73 days at Akrotiri for green turtles, which appear to have slightly longer average incubation durations (average 52 days) than loggerheads with incubation periods ranging from 30 to 71 days at Akrotiri (average 50 days). The loggerhead average incubation duration from earlier years of the project may be less reliable due to less accurate data collection.
The size that females of both species reach at maturity is a source of great variation within populations (Broderick et al., 2003) and has a far-reaching effect on the lifetime reproductive output of a female as they show variation in the number of eggs laid in a clutch and the number of clutches laid in a season. This is one of the reasons that females are measured when possible.
Hatching occurs at Akrotiri from late July until the end of September. From figure 10 it appears that the numbers of live hatchlings are increasing for loggerheads, with the exception of 2003 and 2005. Numbers have increased following a very poor year in 2003 for greens. The difference observed between species is due to the smaller number of nesting greens.
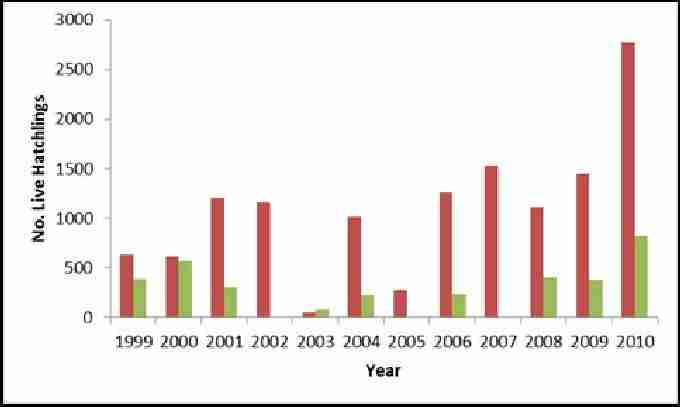
Fig. 10. Showing estimated number of live green and loggerhead hatchlings at Akrotiri 1999-2010. Red bars show estimated total number of live loggerhead hatchlings. Green bars show estimated number of live green hatchlings.
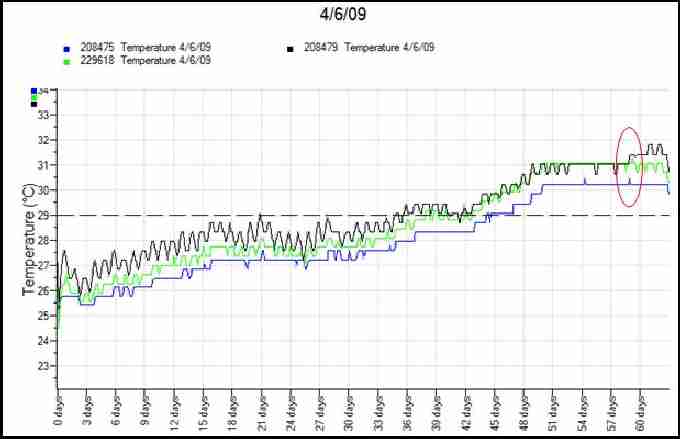
Fig. 11. Temperature profiles from first loggerhead nest of 2009 with data loggers at top (black), middle (green) and bottom (blue) of nest. Dashed line represents pivotal temperature for sex ratio. Oval indicates peak in temperatures.
Figure 11 shows a recorded temperature profile of a loggerhead nest. The red oval highlights the simultaneous peak in all three temperature recordings, suggesting the point the hatchlings emerged from their shells in the nest. Due to the middle third of this incubation taking place below the pivotal sex temperature, it is likely that the nest comprised mostly males. Figure 12 is a temperature profile from the middle of a loggerhead nest in 2008 which was laid particularly close to the high tide line. The areas highlighted in red illustrate temperature drops caused by tidal inundation corresponding with high storm winds, resulting in this nest failing. Turtlewatch tries to relocate nests that are below the average high tide line in order to avoid waterlogging of nests. They are moved as soon as possible after laying to a higher area of the beach.
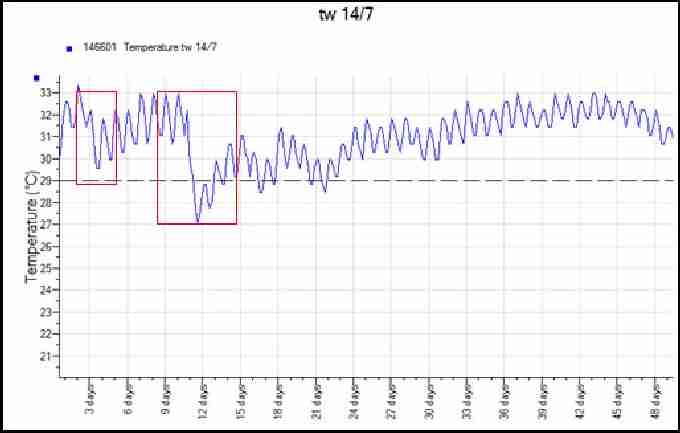
Fig. 12. Temperature profile from a waterlogged loggerhead nest 2008. Red highlighted areas show temperature drops due to water inundation.
Annual data results
As indicated by Tables 1 and 2, green turtles lay less frequently and in smaller numbers than loggerhead turtles at Akrotiri. In the years they nest, greens have consistently higher hatching success rates in comparison with loggerheads (loggerhead mean success rate = 67.3%; green mean success rate = 79.7%), and also larger clutch sizes. The average loggerhead clutch comprised 83.7 eggs, while green clutches on average contained 106.3 eggs.
Table 1. Average nest excavation data for greens at Akrotiri 1999-2010. Early and Late refers to embryo mortality. Infertile categorised by no obvious embryonic development. Dead refers to number of dead hatchlings found in nests. (Original data: Rogers, 2009)
| Year | Excavated
Nests | Mean%
Dead | Mean%
Infertile | Mean%
Early Stage | Mean%
Late Stage | Mean%
Success Rate | Mean
Clutch Size |
|---|
1999
| 5
| 1.69
| 4.24
| 0.64
| 11.86
| 83.37
| 115.2
|
| 2000 | 6 | 0.6 | 3.3 | 1 | 0.8 | 94.1 | 100.5 |
| 2001 | 3 | 0.3 | 11.3 | 3.2 | 2.9 | 79 | 123.3 |
| 2002 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2003 | 1 | 0 | 23.7 | 0 | 0 | 76.2 | 97 |
| 2004 | 2 | 0 | 2.4 | 1.6 | 3.6 | 92.1 | 122 |
| 2005 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2006 | 3 | 1.1 | 7.3 | 5.5 | 3.5 | 81.7 | 95.3 |
| 2007 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2008 | 4 | 3.8 | 8.9 | 2.4 | 8.1 | 76.8 | 106.1 |
| 2009 | 5 | 0.4 | 8.6 | 0.4 | 26.2 | 75.5 | 87.6 |
| 2010 | 12 | 2.58 | 30.58 | 2.41 | 6.25 | 59.68 | 110.58 |
Table 2. Average nest excavation data for loggerheads at Akrotiri 1999-2010. Early and Late refers to embryo mortality. Infertile categorised by no obvious embryonic development. Dead refers to number of dead hatchlings found in nest. Brackets in 2009-10 indicate the number of excavated nests included in analysis in proportion to the total, as depredated nests were not included. (Original data: Rogers, 2009)
| Year | Excavated
Nests | Mean%
Dead | Mean%
Infertile | Mean%
Early Stage | Mean%
Late Stage | Mean%
Success Rate | Mean
Clutch Size |
|---|
1999
| 10
| 1.3
| 8.1
| 1.6
| 1.7
| 86.3
| 69.8
|
| 2000 | 9
| 0.5 | 7.7 | 4.1 | 4.8 | 80.6 | 81 |
| 2001 | 22
| 6.1 | 6.1 | 7 | 17.4 | 67.1 | 87.4 |
| 2002 | 23
| 4.8
| 6.7
| 13.1
| 13.1
| 64.1
| 79.9
|
| 2003 | 4
| 16.6
| 50.3 | 10.8 | 2.3
| 26.2 | 65.6
|
| 2004 | 17
| 7.9
| 11.1
| 15
| 3.2 | 61.5 | 94.3
|
| 2005 | 10
| 1.7
| 50.8 | 5.5
| 10.6 | 39.6
| 82.2
|
| 2006 | 24
| 2.5 | 19.8 | 2.9 | 5.7 | 6.81 | 75.7 |
| 2007 | 33
| 1.6
| 17.5
| 9.1
| 4.1
| 69
| 69.2
|
| 2008 | 22
| 4.5 | 24.7 | 7.1 | 7.7 | 59 | 73.5 |
| 2009 | 35(total 51)
| 1.3
| 24
| 4.5 | 7.9 | 58
| 59.2 |
| 2010 | 37(total 40)
| 6.16 | 19.2 | 2.13 | 11.46 | 61.21 | 83.4 |
Although Bell et al. (2009) found no obvious decline in fertility rates in small populations of either species, there appears to be one for the Akrotiri populations. Figure 13 shows the mean values of eggs in all nests that showed no embryonic development. Nests that were depredated, relocated or waterlogged were not included in this analysis. This average appears to be showing a general increase, which is concerning. However, it is possible that this may be due to a more concerted effort during more recent expeditions to definitively categorise eggs during excavations. Future expeditions will continue to monitor the proportions of eggs which show no embryonic development in order to monitor infertility rates for the Akrotiri populations.
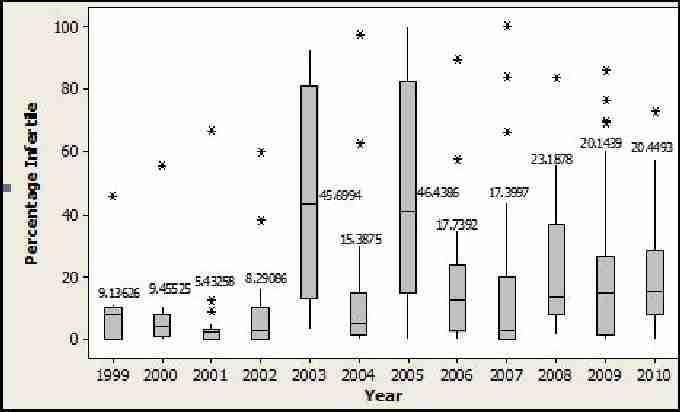
Fig. 13. Boxplot of loggerhead and green mean number of eggs showing no embryonic development 1999-2010. Stars indicate outlying values. Bar in blue box indicates mean value which is also expressed as a numerical value.
Fox Predation
There were persistent problems with fox predation events (Fig. 14) which appears to be a more recent problem. In the 2009 season 40% of nests (n=22) showed some sign of activity around them (Table 3). Some nests were completely destroyed while some were partially dug up and others just ‘investigated’ by the foxes (tracks and small scratches on or around nests). In 2010, 7.5% of loggerhead nests were partially destroyed (n=3). Despite the use of deterrents on the nests and regular beach patrols, fox predation remained a problem. The foxes adapted extremely quickly to any deterrents used and found ways to dig either through or around the protection and destroy some of the nests in between patrols or during the day. The level of disturbance to the nests was much higher for loggerhead nests, most probably as they are generally shallower than those of greens and so are more easily accessible for the foxes.
Table 3. Data on fox predation behaviours 2009 & 2010. *All were loggerhead nests apart from one green turtle nest that was ‘investigated’ in 2009.
| Type of Predator Activity | 2009
Nests % | 2010
Nests % |
|---|
'Investigation' Only
| 8* 12.8
| 0 0
|
Investigation and partially destroyed
| 5 9
| 3 7.5
|
| Investigation and totally destroyed | 10 18.2 | 0 0 |

Fig. 14. Loggerhead nest Cove 4 which was predated despite fox deterrents.
Sex ratio estimations
As previously stated, the pivotal temperature for sex determination in both species in the Mediterranean is 29ºC during the middle third of the incubation period. Figure 11 illustrates that the incubation temperature from the first nest of the season in 2009 did not rise above that threshold. However, 80% of other TDL nests from 2008 and 2009 did exceed it. Therefore a female biased sex ratio has been estimated for the study nests at Akrotiri. This result correlates with other studies in the Mediterranean, Florida and Brazil which suggest female biased sex ratios (Broderick et al., 2001; Kaska et al., 1998; Godley et al., 2001a & b; Marcovaldi et al., 1997; Zbinden et al., 2006; Rees & Margaritoulis, 2004). It is thought that this ratio will become even more skewed, on a global scale, with the increased temperatures resulting from global warming (Weishampel et al., 2004).
Report on dead strandings
From January 2009 until the end of the nesting season, a total of 18 dead turtles washed up on the beaches at Akrotiri. In some cases, it was obvious that fishing lines were the cause of drowning. However, in other cases the cause of death was uncertain. Further research – involving necropsy – would be beneficial to reliably establish cause of death where possible. Turtlewatch encourages people to place value on the lives of turtles but in some cases it is not possible. What this tells us is that our work is far from finished and is still of vital importance if turtle numbers in the Mediterranean are to recover.
Conclusions
Data indicate that although green turtles breed less consistently and in fewer numbers at Akrotiri, they consistently invest a large amount of energy in reproduction and obtain better hatching success rates than loggerheads. Our current data suggest that a female bias sex ratio exists at Akrotiri and further analysis on the Turtlewatch data presented is still being carried out. Information such as the relationship between incubation duration and sex ratio (Rees & Margaritoulis, 2005) at Akrotiri needs more investigation as this could be a useful tool for sex ratio calculations that is less invasive than the within-nest use of data loggers.
Schofield et al. (2009) predict that the Mediterranean basin will experience some of the greatest environmental changes from global climate change. In this scenario, Mediterranean turtle populations would be among the first, on a global scale, to experience climatic changes. Adequate research must be conducted into the mechanisms that determine the sex ratios produced by location, within-season and between-season variations. It is hoped that future Turtlewatch expeditions will be allowed to carry out this research, as by sampling a larger number of clutches and collecting more nest and beach temperature data, a better understanding of temperature and sex variation within clutches – and the Akrotiri population – could be obtained.
We also still need fundamental population data, as it is not known if the Akrotiri nesting populations comprise the same individual females each season, as turtles are not tagged. Further study involving the monitoring of the breeding females with flipper tags or passive integrated transponder tags (PIT) would allow the calculation of remigration intervals for individuals and help to identify new individuals. This will allow basic capture-mark-recapture studies to be established as well as basic population parameters, subsequently allowing better monitoring of the nesting population at Akrotiri and modelling of viable nesting populations. Future expeditions must focus equally on hatchlings and adults in order to establish reliable population demographic data that can be implemented for promoting the recovery of the Mediterranean populations.
Turtlewatch experienced the best season ever in 2009, with regard to nest numbers, with a total of 56 nests. However, fox predation levels appeared higher than in previous years and so subsequent expeditions must ensure that the most effective measures are employed as fox deterrents. The value of long term data, such as that collected by eleven years of Turtlewatch expeditions, cannot be overestimated when dealing with such long-lived species. Long time lags in growth and reproduction may often prevent us from attributing variation in populations to specific detrimental or beneficial actions. Long-term studies concerning the multiple life stages of these turtles are therefore essential in order to adequately document the demographic changes within a given population. Turtlewatch intends to be there to continue our long-term data collection work and to promote the recovery of these fragile populations within their environment.
Acknowledgements
Permission for this research was obtained by application to the Head of the Environmental Department (SBA Administration) and also with permission from the Akrotiri Environmental Centre. Thanks go to all Turtlewatch volunteers, the Glasgow University Exploration Society, RAF Akrotiri for accommodation and feeding us, Staff from Akrotiri Environmental Centre, RAF Turtlewatch committee members, Prof. J.R. Downie for his advice and manuscript editing, my anonymous reviewer for helpful comments and editing and finally to Chief Tech Clive Burt for his dedication and unfailing support. I would also like to thank the BCG for inviting me to talk at their Northern Symposium. Thanks also to all our financial supporters: Glasgow University Court; British Chelonia Group; Carnegie Trust. All photos taken by Sofie Rogers.
References
Ackerman, R.A. (1997). The nest environment and the embryonic development of sea turtles. In: The Biology of Sea Turtles. P. L. Lutz & J.A. Musick. CRC Marine Science Series, CRC Press Inc, Boca Raton, FL, pp. 83-106.
Bell, C.D., Blumenthal, J.M., Broderick, A.C. & Godley, B.J. (2009). Investigating potential for depensation in marine turtles: How low can you go? Conservation Biology 24: 226-235.
Booth, D.T. & Astill, K. (2001). Incubation temperature, energy expenditure and hatchling size in the green turtle (Chelonia mydas), a species with temperature-sensitive sex determination. Australian J. Zool. 49: 389-396.
Booth, D.T., Burgess, E., McCosker, J. & Lanyon, J.M. (2004). The influence of incubation temperature on post-hatchling fitness characteristics of turtles. International Congress Series 1275: 226-233.
Booth, D.T., Burgess, E., McCosker, J. & Lanyon, J.M. (2006). Swimming performance of hatchling green turtles is affected by incubation temperature. Coral Reefs 25: 341-349.
Broderick, A.C., Godley, B.J. & Hays, G.C. (2001). Metabolic heating and the prediction of sex ratios for green turtles (Chelonia mydas). Physiological and Biochemical Zool. 74(2): 161-170.
Broderick, A.C., Glen, F., Godley, B.J. & Hays, G.C. (2003). Variation in reproductive output of marine turtles. J. Exp. Marine Biol. and Ecol. 288(1): 95-109.
Downie, J.R., Muir, H., Dinwoodie, M., McKinnon, L., Dodds, L. & Cascarina, M. (2003). Turtlewatch: A collaboration between Glasgow University and RAF Akrotiri to protect marine turtles in Cyprus. Testudo 5(5): 14-21.
Dial, B.E. (1987). Energetics and performance during nest emergence and the hatchling frenzy in loggerhead sea-turtles (Caretta caretta). Herpetologica 43(3): 307-315.
Glen, F., Broderick, A.C., Godley, B.J. & Hays, G.C. (2003). Incubation environmental affects phenotype of naturally incubated green turtle hatchlings. J. Marine Biol. Assn. of the UK 83: 1183-1186.
Glen, F., Broderick, A.C., Godley, B.J. & Hays, G.C. (2005). Patterns in the emergence of green (Chelonia mydas) and loggerhead (Caretta caretta) turtle hatchlings from their nests. Marine Biology 146: 1039-1049.
Godley, B.J., Broderick, A.C. & Mrosovsky, N. (2001a). Estimating the hatchling sex ratios of loggerhead turtles in Cyprus from incubation durations. Marine Ecology Progress Series 210: 195-201.
Godley, B.J., Broderick, A.C., Downie, J.R., Glen, F., Houghton, J.D., Kirkwood, I., Reece, S. & Hays, G.C. (2001b). Thermal conditions in nests of loggerhead turtles: further evidence suggesting female skewed sex ratios of hatchling production in the Mediterranean. J. Exp. Marine Biol. and Ecol. 263: 45-63.
Kaska, Y., Downie, R., Tippet, R. & Furness, R.W. (1998). Natural temperature regimes for loggerhead and green turtle nests in the eastern Mediterranean. Can. J. Zool. 76: 723-729.
Marcovaldi, M., Godfrey, M. & Mrosovsky, N. (1997). Estimating sex ratios of loggerhead turtles in Brazil from pivotal incubation temperatures. Can. J. Zool. 75: 755-770.
Mickelson, L.E. & Downie, J.R. (2010). Influence of incubation temperature on morphology and locomotion performance of Leatherback (Dermochelys coriacea) hatchlings. Can. J. Zool. 88(4): 359-368.
Nicholls, R.J. & Hoozemans, F.M.J. (1996). The Mediterranean: Vulnerability to coastal implications of climate change. Ocean & Coastal Management 31: 2-3; 105-132.
Rees, A.F. & Margaritoulis, D. (2004). Beach temperatures, incubation durations and estimated hatchling sex ratio for loggerhead sea turtle nests in southern Kyparissia Bay, Greece. Testudo 6(1): 23-36.
Rogers, S.L. (2009). The Effect of Nest Temperature on Hatchling Morphology, Speed and Sex in Green (Chelonia mydas) and Loggerhead (Caretta caretta) Hatchlings in Southern Cyprus. Faculty of Biomedical and Life Sciences – University of Glasgow.
Sales, G., Giffoni, B.B. & Barata, P.C.R. (2008). Incidental catch of sea turtles by the Brazilian pelagic longline fishery. J. Marine Biol. Assn. of the UK 88(4): 853-864.
Schofield, G., Bishop, C.M., Katselidis, K.A., Dimopoulos, P., Pantis, J.D. & Hays, G.C. (2009). Microhabitat selection by sea turtles in a dynamic thermal marine environment. J. Animal Ecol. 78: 14-21.
Tucker, J.K. (2000). Body size and migration of hatchling turtles: inter and intraspecific comparisons. Journal of Herpetology 34(4): 541-546.
Weishampel, J.F., Bagley, D.A. & Ehrhart, L.M. (2006). Intra-annual loggerhead and green turtle spatial nesting patterns. Southeastern Naturalist 5(3): 453-462.
Wibbels, T. (2003). Critical approaches to sex determination in sea turtles. In: The biology of sea turtles Volume II. P.L. Lutz, J.A. Musick & J. Wyneken. CRC Marine Science Series, CRC Press Inc, Boca Raton, FL, pp. 103-134.
Zbinden, J.A., Margaritoulis, D. & Arlettaz, R. (2006). Metabolic heating in Mediterranean loggerhead sea turtle clutches. J. Exp. Marine Biol. and Ecol. 334: 151-157.
Testudo Volume Seven Number Three 2011.
Top